
Page | 1
Community Vaccination
Centers Playbook
February 4, 2021

Page | 2
Record of Changes
This Playbook should be reviewed and updated as necessary.
Change #
Date
Remarks
Table of Contents
Record of Changes ........................................................................................................................... 2
Table of Contents .............................................................................................................................. 2
1.0 Situation ........................................................................................................................................ 3
2.0 Mission and End State ......................................................................................................... 4
3.0 Execution ....................................................................................................................................... 4
4.0 Administration .......................................................................................................................... 16
5.0 Oversight, Coordinating Instructions and Communications ...................... 16
Appendic es .......................................................................................................................................... 17
Appendix A ............................................................................................................................... 18
Appendix B ............................................................................................................................... 19
Appendix C ............................................................................................................................... 26
Appendix D ............................................................................................................................... 32
Appendix E ............................................................................................................................... 37
Appendix F ................................................................................................................................ 38
Appendix G ............................................................................................................................... 39
Acronyms List ......................................................................................................................... 40
Glossary ..................................................................................................................................... 41

Page | 3
1.0 Situation
1.1 Purpose
This playbook establishes guidance for providing federal support to existing and new Community Vaccination
Centers (CVCs) that are essential to accomplishing the mission, to include interagency coordination, resource
support, facility setup, and other requirements that may necessitate federal support.
1.2. Background
To date, the ongoing COVID-19 pandemic has claimed the lives of more than 430,000 Americans. While
mitigation measures such as social distancing and the wearing of masks are effective tools in preventing the
spread of COVID-19, an additional way to protect people and reduce the spread of this disease is with the
widespread administration of COVID-19 vaccines. As part of a national effort to speed the pace of COVID-19
vaccination campaigns, the President has directed the federal government to establish new federally supported
CVCs. As stated in
the National Strategy for COVID-19 Response and Pandemic Preparedness, FEMA is charged
with supporting the set-up and operations of such CVCs.
1.3. Assumptions
• Multiple federal agencies are able to supply or support states, tribes, and territories (STT) staffing
augmentation needs, based on authorization and identified staffing capability to support clinical
and/or non-clinical requirement (e.g. vaccine administration vs. general crowd management and
administrative support)
• There will be a change in the available national vaccine supply, storage requirements for vaccine
centers, and the number of doses required by recipients pending vaccine developments
• Plans for operating and activating CVCs must be coordinated with STT authorities to support access to
vaccination in jurisdictions
• Staffing requirements may change as a function of the facility or location
1.4. Critical Considerations
• Distribution processes for vaccines and supplies critical to administration vary depending on
manufacturer and jurisdiction
• Supply chain constraints due to the pandemic may lead to unanticipated challenges procuring
supplies necessary for facility setup
• Vaccine administration timelines and required doses vary depending on manufacturer and the
applicable FDA-issued vaccine EUAs
• CVCs should have the capability to collect, organize, and store information if unable to access digital
system platforms for vaccine administration
• The Regional Response Coordination Center (RRCC) must work with STT to develop plans that address
vaccination of homebound residents, those with limited access to transportation, mobility limitations,
etc.
• Planning for distribution of vaccine to members of Tribes must be coordinated with all the appropriate
entities, including but not limited to FEMA regions, the Bureau of Indian Affairs (BIA) and/or Indian
Health Service (IHS) to develop specific plans for direct IHS distribution and facilitate administration
of vaccines to IHS Direct, Tribal Health Programs and Urban Indian Organizations who elected to
receive vaccines through the BIA or IHS
• All jurisdiction COVID-19 mitigation mandates must be adhered to by staff and vaccine recipients
(mask wearing, social distancing, washing hands/use of hand sanitizer)
• At a minimum, all COVID-19 mitigation mandates from CDC’s
Interim Infection Prevention and Control
Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19)
Pandemic must be adhered to. Jurisdictions may require mitigation measures in addition to these
• If a facility is federally controlled, it must meet all DHS and FEMA requirements for facility access and
physical security in accordance with Federal policy and guidelines
• The STT ultimately have the authority to choose to set licensure/certification requirements for
vaccinators working at their direction. However, STT should be advised that Declarations under the
PREP Act for COVID-19 have vastly expanded the available pool of potential vaccinators, through the

Page | 4
preemption of state laws under these declarations. STT should be encouraged, to the maximum extent
possible, to utilize this expanded authority to use non-traditional vaccinators, available through the
PREP Act
1.5. Limiting Factors
• Current supply of COVID-19 vaccine does not meet national demand
• Medical consumables and products in support of the vaccine administration may be limited
• CVCs storage and management of vaccine supplies
o Cold-chain storage and handling requirements for each COVID-19 vaccine product will vary
from refrigerated (2°C to 8°C) to frozen (-15°C to -25°C) to ultra-cold (-60°C to -80°C)
temperatures, and ongoing stability testing may impact these requirements
o Cold-chain storage equipment is not necessarily available at all traditional vaccine
administration CVCs
• Public health and medical personnel are a scarce resource (especially physicians, nurses, respiratory
technicians, laboratory technicians, and emergency medical services staff/personnel)
• STT partners are utilizing different processes of varying sophistication for information management
and vaccine recipients may not understand the registration process nor how to ask for an
accommodation if the state is providing language services
• Effective communication access may be limited to virtual connections as in-person support is limited.
Virtual connectivity may be limited in some areas
• Availability of staff critical to facility selection and setup is limited (Disability Integration, Equal Rights,
External Affairs)
• Medical waste disposal requirements will vary by jurisdiction
2.0 Mission and End State
2.1. Mission
Provide support such as set up, equipment, information management, staffing, and CVC operation to existing
or new CVCs including mobile clinics in STT areas leveraging close coordination between the federal
government and all vaccination jurisdictions to foster timely and equitable distribution and administration of
COVID-19 vaccines.
2.2. End State
STT have a sustainable capability to administer vaccinations now and in the future
3.0 Execution
3.1 Operations
3.1.1. Operational Approach
The federal government will support STT vaccination programs by providing resources for pre-existing facilities
and/or establishing new federally operated facilities. Facilities will be established as fixed facility, drive-
through facility, or as a mobile vaccination clinic (See Appendix A for additional information on CVC types). Site
selection for CVCs will be needs based, data driven, and in support of STT requests. The objective of both
federally supported and federally operated CVCs is to maximize the timely and safe administration of the
vaccine to all recipients. Facility size models will be based on throughput over a 12-hour shift and are as
following:

Page | 5
3.1.2. Fixed Facility (Walk-in) Checklist
The list of actions below facilitates the effective and efficient administration of vaccinations in a walk-in, fixed
facility. These actions are formatted as a checklist but many of the actions will be initiated concurrently, not
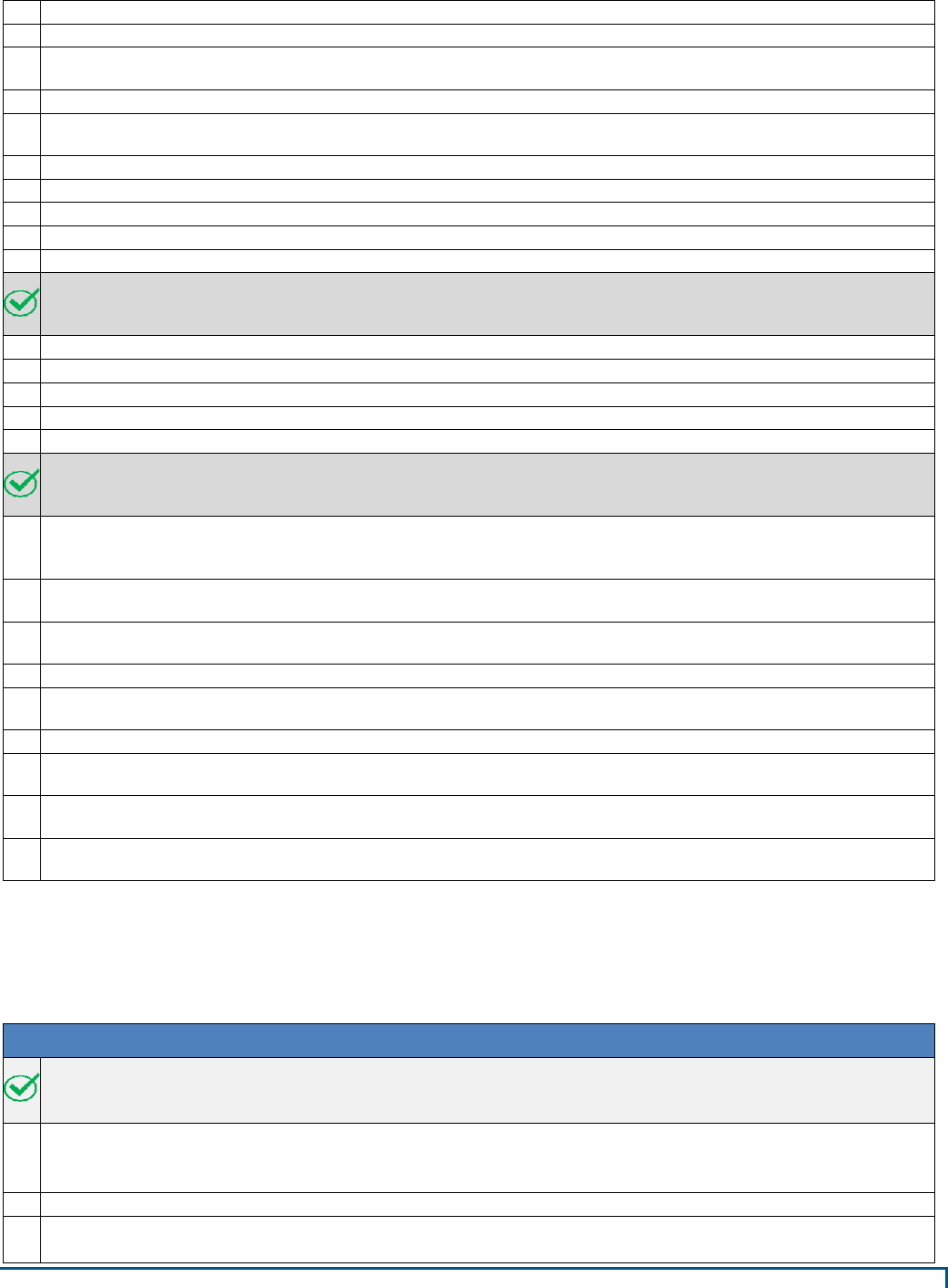
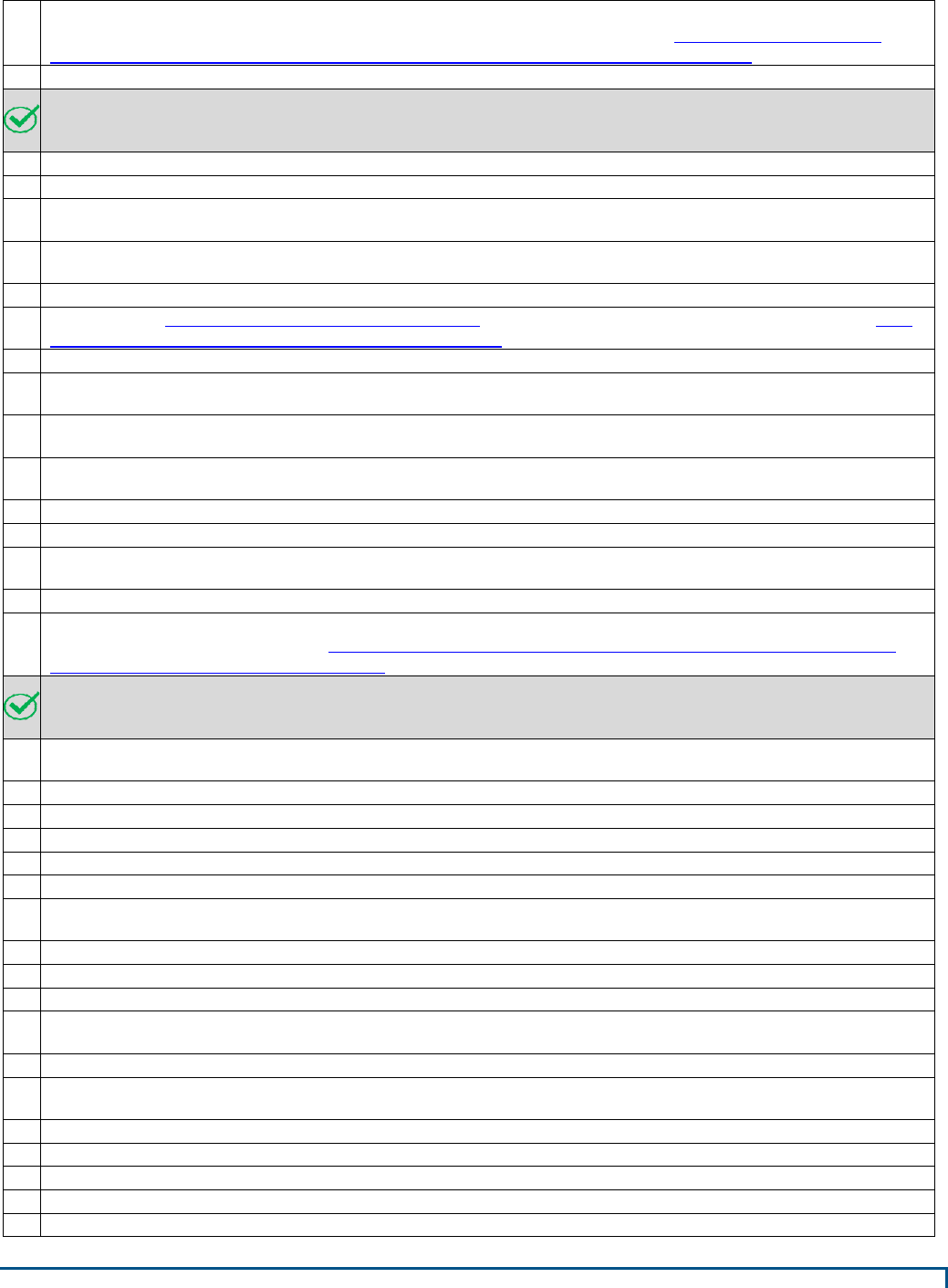
sequentially. See Appendix B for conceptual layouts of the facility types.
Fixed Facility
Selection Actions
If location not already identified by STT and approved by the region, conduct a search and sourcing
process using either FEMA Logistics or General Services Administration (GSA) for site selection. Use
Civil Rights Checklist (Appendix C) to ensure equity
Conduct vaccination site assessment (key participants: local public health officials, Safety, Security,
Civil Rights, Emergency Management Officials, Fire Inspector & Office of Disability Integration
Coordination)
Using the FEMA Disaster Facility Setup Guide and Disaster Facility Setup Guide Updates or other
appropriate criteria, support the jurisdiction in determining the spacing and layout needs for the
required CVC
Coordinate the appropriate license and space utilization agreement (LUA) and/or memorandum of
understanding (MOUs)
Ensure adequate traffic control plan, set-up space, and staging areas to accommodate operations
Confirm communication lines (landline/cellphone and computer/internet) are operational and
accessible for people with disabilities as required with mobile wireless access points (MiFi’s/Cradle
points)
Identify a location for stand-by ambulance at the CVC for management of recipients with on-site
medical emergencies
Identify pre-solicited, signed and or other standing agreements – either federal, state or local that can
be extended in order to provide janitorial/custodial services. Also establish agreements for medical
waste disposal services
Coordinate with local authorities for on-site security, public transportation to the CVC, outreach, and
other community impact considerations and requirements
All CVCs should have emergency backup power to the storage equipment of the vaccine supply. This
emergency power will ensure continuous cold storage in the event of a Public Safety Power Shutoff
(PSPS), or storm interrupts the local electrical power supply
Ensure location of the facility is added as an approved site in the CDC's Vaccine Tracking System
(VTrckS) to enable ordering and delivery of vaccine to the CVC, have a signed CDC provider agreement,
and have Vaccine Finder sign up for vaccine dose tracking
Review training plan for all staff and each required role as established by the STT
Ensure facility opening dates are communicated to the public
Develop a strategy for demobilization of the CVC or transfer of operation from Federal to STT
Pre-Clinical Actions
Facilitate and coordinate the Resource Request Form (RRF) process for federally supported mission
assignments to include staffing, contracting, and other resource requirements for the receiving site via
the FEMA Regional Response Coordination Center (RRCC) in consultation with ESF8: Health and Human
Services (HHS) Office of the Assistance Secretary for Preparedness and Response (ASPR)
Coordinate with the STT to determine how much vaccine allocation the CVC should expect from the STT
allocations of the vaccine
Facility Size Models (for new facilities)
Type 1
Approximate capacity
of
6,000 doses a day
Minimum of 15,000 sf
with adequate parking
for at least 800
vehicles
Type 2
Approximate capacity
of
3,000 doses a day
Minimum of 7,500 sf
with adequate
parking
for at least 600
vehicles
Type 3
Approximate capacity
of
1,000 doses a day
Minimum of 4,500 sf
with adequate parking
for at least 250
vehicles
Type 4
Approximate capacity
of
250 doses a day
Minimum of 2,500 sf
with adequate parking
for at least 130
vehicles
Type 5 – Mobile Site
Approximate capacity
of
250 doses a day
Minimum of 2,500 sf
with
adequate parking
for at least 130
vehicles

Page | 6
Coordinate with the jurisdiction to determine the type and the required throughput capacity of the CVC.
(The number of persons preregistered in the receiving jurisdiction may be useful to estimate throughput
needs)
Confirm if federal support is for an existing community vaccination center or a new CVC that needs to be
established
Coordinate with jurisdiction to determine community requirements (urban, suburban, rural, remote) for
vaccination CVCs (fixed, mobile, drive-through)
Coordinate with the jurisdiction to identify access and functional needs required at the CVC for potential
vaccine recipients, to include sign language, captioning services, Braille, large print, and translation and
interpreting for non-English users
Review CDC’s Vaccine Storage and Handling Toolkit and FDA’s appropriate manufacture vaccine’s Fact
Sheet for Healthcare Providers Administering Vaccine to ensure adequate storage is available on-site or
if transportation will be required to bring the vaccine dosages to the CVC each day. Ensure vaccines
were transported appropriately
Ensure the vaccine allocation for the CVC will adequately support desired throughput for the day
Review receiving jurisdiction regulations governing the practice of health care professionals. (This
should be considered when determining clinic staffing and assignment of roles and responsibilities)
Coordinate with the jurisdiction to determine access requirements, permissions, and training for
required data systems for vaccine administration and distribution tracking
Coordinate with the jurisdiction to determine vaccine allocation with receiving jurisdiction to include the
quantity, type, and storage/handling requirements at the CVC
Coordinate with the jurisdiction to ensure contingency plan is developed and in place if vaccinations are
compromised and/or need replacement
Ensure the medical screener discusses with potential vaccine recipients to identify
persons with contraindications and precautions. Ensure staff follow CDC’s Interim Considerations:
Preparing for the Potential Management of Anaphylaxis After COVID-19 Vaccination
Stage the ALS ambulance at an appropriate location to be readily accessible to the whole facility
Clinical Daily Operational Actions
Ensure minimum staffing and work assignments and schedule is established for the day
Confirm vaccine inventory is on-site to meet the expected throughput for the day
Pre-screening of CVC staff is accomplished using temperature screening and symptom and exposure
questionnaire
Ensure appropriate quantity of PPE is staged and available for CVC staff based on anticipated daily burn
rate. Ensure all staff have and utilize their PPE at all times
Ensure availability of appropriate medical consumables based on the anticipated daily burn rate
Follow CDC’s Vaccine Storage and Handling Toolkit and FDA’s appropriate manufacture vaccine’s Fact
Sheet for Healthcare Providers Administering Vaccine
Ensure appropriate amount of sanitation and work surface disinfectant supplies
Ensure appropriate amount of medical documentation (intake forms, etc.) and has adequate locked
storage areas
Establish a process to verify the arriving potential vaccine recipients have an appointment that day to
receive a vaccine
Assign appropriate staff to the Recipient Exit Area/Exit Reviewer in order to observe recipients for
adverse reactions to vaccine
Establish a staging area to address any additional needs
Ensure effective communication to facility support staff to track and monitor medical supplies
Fill out all relevant information on the recipient’s COVID-19 Vaccination Record Card and record the date
and vaccine lot number and schedule the second vaccine shot (if applicable)
Sanitize the vaccine administration work area after each vaccine administration
Send the recipient to the observation area to wait for the described post-vaccination waiting time per the
CDC guidelines outlined in CDC’s Interim Considerations: Preparing for the Potential Management of
Anaphylaxis After COVID-19 Vaccination
Facility Support Daily Operational Actions

Page | 7
Conduct a pre-opening facility sweep to ensure that all safety and sanitization procedures have been
followed and are in place
Ensure minimum staffing, work assignments, and schedule is established for the day
Ensure traffic/access control process is in place for the facility and the parking lot
Conduct a Daily Shift/Safety Briefing with all CVC staff prior to opening the CVC
Establish the day’s battle rhythm and ensure all CVC staff are aware of it
Review and understand EEIs and other reporting requirements for all appropriate entities
Ensure signage is posted that describes the vaccine recipient flow starting from outside the facility
including the Check-In/Screening Area, and all the way to Observation Area
Ensure appropriate information technology (IT) support is available
Stage Pre-Waiting Area where vaccine recipients wait to be sent to a vaccination station
Ensure an area is set aside for staff to take allotted break(s)
Verify all personnel are in place and all stations are ready to process vaccine recipients prior to opening
the facility
Ensure a process is in place for regular disinfecting of the CVC
Pre-screening of vaccine recipients at the Check-In/Screening Area using a temperature screening and
symptom and exposure questionnaire
Ensure process is in place to monitor and track facility supplies and track daily burn rates
Monitor occupancy levels in the observation area to prevent over crowding
Establish a staff accountability process to include a sign in and sign out process
Ensure a process is in place for proper handling/disposal of medical waste
Ensure a process is in place for general facility waste handling
Facility End of Shift Actions
Conduct an end of day supervisor meeting with relevant staff
Ensure all remaining vaccines are adequately secured and stored for the night
Thoroughly sanitize all workstations and public areas
Ensure all medical records (PII documents) are appropriately secured and stored
Ensure CVC location is fully secured prior to departure
Facility Close-out/Demobilization Actions
Coordinate with jurisdiction to complete a post-CVC evaluation and ensure post-CVC reporting and
recording o
f vaccinations administered are provided to the jurisdiction immunization information system
(IIS)
Create or reform a demobilization/transition plan upon rightsizing/closing facilities or transferring the
CVC to another organization/agency
Close-out of all support contracts that were supporting the CVC and coordinate the transfer of the
contract over to STT if necessary
Establish a plan for the removal of all equipment and any mitigation for small damage to the facility
Complete final walk-through of the facility with the facility owner in order to secure release of liability and
document condition of the facility upon departure
Ensure the RRCC has reviewed reimbursement requests, paid all bills, and de-obligate funds
Ensure the closeout of a Mission Assignment (MA) at the incident management (IM) and incident
support (IS) levels according to RRCC defined process
Ensure that a plan has been developed to right size or retrograde of Federal resources at the CVC as
needed
Ensure CVC closing dates are communicated by the Public Information Officers to the public if the CVC is
not transitioned to STT management
3.1.3. Drive Through Facility Operational Checklists
The list of actions below facilitates the effective and efficient administration of vaccinations in a drive through.
These actions are formatted as a checklist but many of the actions will be initiated concurrently, not
sequentially. See Appendix B for conceptual layouts of the facility types.

Page | 8
Drive Through Facility
Selection Actions
If location not already identified by STT and approved by the Region, conduct a search and sourcing
process using either FEMA Logistics or General Services Administration (GSA) for site selection. Use
Civil Rights Checklist (Appendix C) to ensure equity
Conduct vaccination site assessment (key participants: Local Public Health Officials, Safety,
Security, Civil Rights, Emergency Management Officials, Fire Inspector & Office of Disability
Integration Coordination)
Using the FEMA Disaster Facility Setup Guide and Disaster Facility Setup Guide Updates or other
appropriate criteria, support the jurisdiction in determining the spacing and layout needs for the
required CVC
Coordinate the appropriate license and space utilization agreement (LUA) and/or memorandum of
understanding (MOUs)
Ensure adequate traffic control plan, set-up space, and staging areas to accommodate operations
Confirm communication lines (landline/cellphone and computer/internet) are operational with mobile
wireless access points (MiFi’s/Cradle points)
Identify a location for stand-by ambulance at the CVC for management of recipients with on-site
medical emergencies
Identify pre-solicited, signed and or other standing agreements – either federal, state, or local that can
be extended in order to provide janitorial/custodial services. Also establish agreements for medical
waste disposal services
Coordinate with local authorities for on-site security, public transportation to the CVC, outreach, and
other community impact considerations and requirements
All CVC facilities should have emergency backup power to the storage equipment of the vaccine
supply. This emergency power will ensure continuous cold storage in the event of a Public Safety
Power Shutoff (PSPS), or storm interrupts the local electrical power supply
Add the location of the facility as an approved site in the CDC's Vaccine Tracking System (VTrckS) to
enable ordering and delivery of vaccine to the CVC
Review training plan for all staff and each required role as established by the STT
Ensure adequate spacing allowance for social distancing from entry to exit
Ensure warming and cooling stations are established for staff with adequate storage for PPE, vaccines,
and other supplies
Ensure facility opening dates are communicated to the public
Develop a strategy for demobilization of the CVC or transfer of operation from Federal to STT
Pre-Clinical Actions
Facilitate and coordinate the Resource Request Form (RRF) process for federally supported mission
assignments to include staffing, contracting, and other resource requirements for the receiving CVC via
the FEMA Regional Response Coordination Center (RRCC) in consultation with ESF8: Health and Human
Services (HHS) Office of the Assistance Secretary for Preparedness and Response (ASPR)
Coordinate with the STT to determine how much vaccine allocation to the CVC should expect from the
STT allocations of the vaccine
Coordinate with the jurisdiction to determine the type and the required throughput capacity of the CVC.
(The number of persons preregistered in the receiving jurisdiction may be useful to estimate throughput
needs)
Confirm if Federal support is for an existing community vaccination center or a new CVC that needs to be
established
Coordinate with jurisdiction to determine community requirements (urban, suburban, rural, remote) for
vaccination CVCs (fixed, mobile, drive-through)
Coordinate with the jurisdiction to identify any additional access and functional needs required at the
CVC for potential vaccine recipients, to include (Language access, including sign language, captioning
services, Braille, large print to provide access to services; language access for translation and
interpreting for non-English users)

Page | 9
Review CDC’s Vaccine Storage and Handling Toolkit and FDA’s appropriate manufacture vaccine’s Fact
Sheet for Healthcare Providers Administering Vaccine to ensure adequate storage is available on-site or
if transportation will be required to bring the vaccine dosages to the CVC each day. Ensure vaccines
were transported appropriately
Ensure the vaccine allocation for the CVC will adequately support desired throughput for the day
Review receiving jurisdiction regulations governing the practice of health care professionals. (This
should be considered when determining clinical staffing and assignment of roles and responsibilities)
Coordinate with the jurisdiction to determine access requirements, permissions, and training for
required data systems for vaccine administration and distribution tracking
Coordinate with the jurisdiction to determine vaccine allocation with receiving jurisdiction to include the
quantity, type, and storage/handling requirements at the CVC
Coordinate with the jurisdiction to ensure contingency plan is developed and in place if vaccinations are
compromised and/or need replacement
Ensure the medical screener discusses with potential vaccine recipients to identify persons with
contraindications and precautions. Ensure staff follow CDC’s Interim Considerations: Preparing for the
Potential Management of Anaphylaxis After COVID-19 Vaccination
Stage the ALS ambulance at an appropriate location to be readily accessible to the whole facility
Clinical Daily Operational Actions
Ensure minimum staffing and work assignments and schedule is established for the day
Confirm vaccine inventory is on-site to meet the expected throughput for the day
Pre-screening of CVC staff is accomplished using temperature screening and symptom and exposure
questionnaire
Ensure appropriate quantity of PPE is staged and available for CVC staff based on anticipated daily burn
rate. Ensure all staff have and utilize their PPE at all times
Ensure availability of appropriate medical consumables based on the anticipated daily burn rate.
Follow CDC’s Vaccine Storage and Handling Toolkit and FDA’s appropriate manufacture vaccine’s Fact
Sheet for Healthcare Providers Administering Vaccine
Ensure appropriate amount of sanitation and work surface disinfectant supplies
Ensure appropriate amount of medical documentation (intake forms, etc.) and has adequate locked
storage areas
Establish a process to verify the arriving potential vaccine recipients have an appointment that day to
receive a vaccine
Assign appropriate staff to the Recipient Exit Area/Exit Reviewer in order to observe recipients for
adverse reactions to vaccine
Establish a staging area to address any additional needs
Ensure effective communication to facility support staff to track and monitor medical supplies
Fill out all relevant information on the recipient’s COVID-19 Vaccination Record Card and record the date
and vaccine lot number and schedule the second vaccine shot (if applicable)
Sanitize the vaccine administration work area after each vaccine administration
Send the recipient to the observation area to wait for the described post-vaccination waiting time per the
CDC guidelines outlined in CDC’s Interim Considerations: Preparing for the Potential Management of
Anaphylaxis After COVID-19 Vaccination
Facility Support Daily Operational Actions
Conduct a pre-opening facility sweep to ensure that all safety and sanitization procedures have been
followed and are in place
Ensure minimum staffing, work assignments, and schedule is established for the day
Ensure traffic/access control process is in place for the facility and the parking lot
Conduct a Daily Shift/Safety Briefing with all CVC staff prior to opening the CVC
Establish the day’s battle rhythm and ensure all CVC staff are aware of it
Review and understand EEIs and other reporting requirements for all appropriate entities
Ensure signage is posted that describes the vaccine recipient flow starting from outside the facility
including the Check-In/Screening Area, and all the way to Observation Area
Ensure appropriate information technology (IT) support is available
Page | 10
Stage Pre-Waiting Area where vaccine recipients wait to be sent to a vaccination station
Ensure an area is set aside for staff to take allotted break(s)
Verify all personnel are in place and all stations are ready to process vaccine recipients prior to opening
the facility
Ensure a process is in place for regular disinfecting of the CVC
Pre-screening of vaccine recipients at the Check-In/Screening Area using a temperature screening and
symptom and exposure questionnaire
Ensure process is in place to monitor and track facility supplies and track daily burn rates
Monitor occupancy levels in the observation area to prevent over crowding
Establish a staff accountability process to include a sign in and sign out process
Ensure a process is in place for proper handling/disposal of medical waste
Ensure a process is in place for general facility waste handling
Facility End of Shift Actions
Conduct an end of day supervisor meeting with relevant staff
Ensure all remaining vaccines are adequately secured and stored for the night
Thoroughly sanitize all workstations and public areas
Ensure all medical records (PII documents) are appropriately secured and stored
Ensure CVC location is fully secured prior to departure
Facility Close-out/Demobilization Actions
Coordinate with jurisdiction to complete a post-CVC evaluation and ensure post-CVC reporting and
recording of vaccinations administered are provided to the jurisdiction immunization information system
(IIS)
Create or reform a demobilization/transition plan upon rightsizing/closing facilities or transferring the
CVC to another organization/agency
Close-out of all support contracts that were supporting the CVC and coordinate the transfer of the
contract over to STT if necessary
Establish a plan for the removal of all equipment and any mitigation for small damage to the facility
Complete final Walk-through the facility with the facility owner in order to secure release of liability and
document condition of the facility upon departure.
Ensure the RRCC has reviewed reimbursement requests, paid all bills, and de-obligate funds
Ensure the closeout of a Mission Assignment (MA) at the incident management (IM) and incident
support (IS) levels according to RRCC defined process
Ensure that a plan has been developed to right size or retrograde of Federal resources at the CVC as
needed
Ensure CVC closing dates are communicated by the Public Information Officers to the public if the CVC is
not transitioned to STT management
3.1.4. Mobile Vaccination Clinic Operational Checklist
The list of actions below facilitates the effective and efficient administration of vaccinations in a mobile
vacation clinic. These actions are formatted as a checklist but many of the actions will be initiated
concurrently, not sequentially. See Appendix B for conceptual layouts of the facility types.
Mobile Vaccination Clinic
Selection Actions
If location not already identified by STT and approved by the Region, conduct a search and sourcing
process using FEMA Logistics for site selection. Use Civil Rights Checklist (Appendix C to ensure
equity
Ensure that parking area is assessed for safety and accessibility
Conduct vaccination site assessment (key participants: Local Public Health Officials, Safety,
Security, Civil Rights, Emergency Management Officials, Fire Inspector & Office of Disability

Page | 11
Integration Coordination)
Using the FEMA Disaster Facility Setup Guide and Disaster Facility Setup Guide Updates or other
appropriate criteria, support the jurisdiction in determining the spacing and layout needs for the
required CVC
Coordinate the appropriate license and space utilization agreement (LUA) and/or memorandum of
understanding (MOUs)
Ensure adequate traffic control plan, set-up space, and staging areas to accommodate operations
Confirm communication lines (landline/cellphone and computer/internet) are operational and
accessible for people with disabilities as required with mobile wireless access points
(MiFi’s/Cradle points)
Identify a location for stand-by ambulance at the CVC for management of recipients with on-site medical
emergencies
Identify pre-solicited, signed and or other standing agreements – either federal, state, or local that can
be extended in order to provide janitorial/custodial services. Also establish agreements for medical
waste disposal services
Coordinate with local authorities for on-site security, public transportation to the CVC, outreach and
other community impact considerations and requirements
All CVCs should have emergency backup power to the storage equipment of the vaccine supply. This
emergency power will ensure continuous cold storage in the event of a Public Safety Power Shutoff
(PSPS), or storm interrupts the local electrical power supply
Add the location of the facility as an approved site in the CDC's Vaccine Tracking System (VTrckS) to
enable ordering and delivery of vaccine to the CVC
Review training plan for all staff and each required role as established by the STT
Ensure facility opening dates are communicated to the public
Develop a strategy for demobilization of the CVC or transfer of operation from Federal to STT
Pre-Clinical Actions
Facilitate and coordinate the Resource Request Form (RRF) process for federally supported mission
assignments to include staffing, contracting, and other resource requirements for the receiving CVC via
the FEMA Regional Response Coordination Center (RRCC) in consultation with ESF8: Health and Human
Services (HHS) Office of the Assistance Secretary for Preparedness and Response (ASPR)
Coordinate with the STT to determine how much vaccine allocation to the CVC should expect from the
STT allocations of the vaccine
Coordinate with the jurisdiction to determine the type and the required throughput capacity of the CVC.
(The number of persons preregistered in the receiving jurisdiction may be useful to estimate throughput
needs)
Confirm if Federal support is for an existing community vaccination center or a new CVC that needs to be
established
Coordinate with jurisdiction to determine community requirements (urban, suburban, rural, remote) for
vaccination CVCs (fixed, mobile, drive-through)
Coordinate with the jurisdiction to identify any additional access and functional needs required at the
CVC for potential vaccine recipients, to include (Language access, including sign language, captioning
services, Braille, large print to provide access to services; language access for translation and
interpreting for non-English users)
Review CDC’s Vaccine Storage and Handling Toolkit and FDA’s appropriate manufacture vaccine’s Fact
Sheet for Healthcare Providers Administering Vaccine to ensure adequate storage is available on-site or
if transportation will be required to bring the vaccine dosages to the CVC each day. Ensure vaccines
were transported appropriately
Ensure the vaccine allocation for the CVC will adequately support desired throughput for the day
Review receiving jurisdiction regulations governing the practice of health care professionals. (This
should be considered when determining clinical staffing and assignment of roles and responsibilities)
Coordinate with the jurisdiction to determine access requirements, permissions, and training for
required data systems for vaccine administration and distribution tracking
Coordinate with the jurisdiction to determine vaccine allocation with receiving jurisdiction to include the
quantity, type, and storage/handling requirements at the CVC
Coordinate with the jurisdiction to ensure contingency plan is developed and in place if vaccinations are
compromised and/or need replacement
Page | 12
Ensure the medical screener discusses with potential vaccine recipients to identify
persons with contraindications and precautions. Ensure staff follow CDC’s Interim Considerations:
Preparing for the Potential Management of Anaphylaxis After COVID-19 Vaccination
Stage the ALS ambulance at an appropriate location to be readily accessible to the whole facility
Clinical Daily Operational Actions
Ensure minimum staffing and work assignments and schedule is established for the day
Confirm vaccine inventory is on-site to meet the expected throughput for the day
Pre-screening of CVC staff is accomplished using temperature screening and symptom and exposure
questionnaire
Ensure appropriate quantity of PPE is staged and available for CVC staff based on anticipated daily burn
rate. Ensure all staff have and utilize their PPE at all times
Ensure availability of appropriate medical consumables based on the anticipated daily burn rate
Follow CDC’s Vaccine Storage and Handling Toolkit and FDA’s appropriate manufacture vaccine’s Fact
Sheet for Healthcare Providers Administering Vaccine
Ensure appropriate amount of sanitation and work surface disinfectant supplies
Ensure appropriate amount of medical documentation (intake forms, etc.) and has adequate locked
storage areas
Establish a process to verify the arriving potential vaccine recipients have an appointment that day to
receive a vaccine
Assign appropriate staff to the Recipient Exit Area/Exit Reviewer in order to observe recipients for
adverse reactions to vaccine
Establish a staging area to address any additional needs
Ensure effective communication to facility support staff to track and monitor medical supplies
Fill out all relevant information on the recipient’s COVID-19 Vaccination Record Card and record the date
and vaccine lot number and schedule the second vaccine shot (if applicable)
Sanitize the vaccine administration work area after each vaccine administration
Send the recipient to the observation area to wait for the described post-vaccination waiting time per the
CDC guidelines outlined in CDC’s Interim Considerations: Preparing for the Potential Management of
Anaphylaxis After COVID-19 Vaccination
Facility Support Daily Operational Actions
Conduct a pre-opening facility sweep to ensure that all safety and sanitization procedures have been
followed and are in place
Ensure minimum staffing, work assignments, and schedule is established for the day
Ensure traffic/access control process is in place for the facility and the parking lot
Conduct a Daily Shift/Safety Briefing with all CVC staff prior to opening the CVC
Establish the day’s battle rhythm and ensure all CVC staff are aware of it
Review and understand EEIs and other reporting requirements for all appropriate entities
Ensure signage is posted that describes the vaccine recipient flow starting from outside the facility
including the Check-In/Screening Area, and all the way to Observation Area
Ensure appropriate information technology (IT) support is available
Stage Pre-Waiting Area where vaccine recipients wait to be sent to a vaccination station
Ensure an area is set aside for staff to take allotted break(s)
Verify all personnel are in place and all stations are ready to process vaccine recipients prior to opening
the facility
Ensure a process is in place for regular disinfecting of the CVC
Pre-screening of vaccine recipients at the Check-In/Screening Area using a temperature screening and
symptom and exposure questionnaire
Ensure process is in place to monitor and track facility supplies and track daily burn rates
Monitor occupancy levels in the observation area to prevent over crowding
Establish a staff accountability process to include a sign in and sign out process
Ensure a process is in place for proper handling/disposal of medical waste
Ensure a process is in place for general facility waste handling

Page | 13
Facility End of Shift Actions
Conduct an end of day supervisor meeting with relevant staff
Ensure all remaining vaccines are adequately secured for the night
Thoroughly sanitize all workstations and public areas
Ensure all medical records (PII documents) are appropriately secured and stored
Ensure CVC location is fully secured prior to departure
Facility Close-out/Demobilization Actions
Coordinate with jurisdiction to complete a post-CVC evaluation and ensure post-CVC reporting and
recording of vaccinations administered are provided to the jurisdiction immunization information system
(IIS)
Create or reform a demobilization/transition plan upon rightsizing/closing facilities or transferring the
CVC to another organization/agency
Close-out of all support contracts that were supporting the CVC and coordinate the transfer of the
contract over to STT if necessary
Establish a plan for the removal of all equipment and any mitigation for small damage to the facility
Complete final walk-through the facility with the facility owner in order to secure release of liability and
document condition of the facility upon departure.
Ensure the RRCC has reviewed reimbursement requests, paid all bills, and de-obligate funds
Ensure the closeout of a Mission Assignment (MA) at the incident management (IM) and incident
support (IS) levels according to RRCC defined process
Ensure that a plan has been developed to right size or retrograde of Federal resources at the CVC as
needed
Ensure CVC closing dates are communicated by the Public Information Officers to the public if the CVC is
not transitioned to STT management
3.1.5. Responsibilities
The Regional Response Coordination Centers will delegate the following responsibilities to the CVCs:
• Resource accountability and tracking to inform resource request and allocations
• Upon the identification of an individual with disability or limited English proficient provide appropriate
contact information or resource to ensure effective communication access and meaningful access to
information
• Tactical control of all resources assigned to the CVCs
• Work assignment development for all assigned resources
• Maintenance and knowledge of the Community Vaccination Center Continuity of Operations (COOP),
Communications Plan and Organizational Chart
• Situational awareness and information reporting
• Other authorities deemed appropriate by the RRCC
3.1.6. Operational Strategy
Operational strategy development and implementation is a shared responsibility between the RRCC and the
CVCs. In short, the Vaccination Task Force is responsible for developing the overarching strategy, whereas the
Clinic Manager is responsible for task organization to implement that strategy. Specific responsibilities are
identified below:
Regional Response Coordination Center Responsibilities
The RRCC has the primary responsibility for directing the operational strategic approach to accomplish the end
state. The RRCC also receives input from the CVCs to contextualize and validate existing priorities and strategies.
Community Vaccination Center Responsibilities
The CVCs have responsibilities for task organizing and prioritizing internal resources to implement the strategy
to meet the throughput requirement. Reporting requirements are due daily to the RRCC by close of business.

Page | 14
3.1.7. Resource Coordination and Management
Clinical, facility support, and administrative staff will be assigned to the CVCs. Staffing requests will be
coordinated through the RRCC via established processes. Clinic Managers are responsible for tracking
demobilization and leave dates and ensuring requests are made with adequate time for the transition of
responsibilities. Staffing requirements will be defined through the RRF process and disseminated to the
appropriate supply sources for fulfillment based on capability and capacity. Force packages or single
resources will deploy to provide the critical staffing support identified by STT community vaccination
operations. These support staff will adhere to current guidance and standards of practice included in the
COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations
. Variations may exist and/or
specialties may be added according to the type and scale of vaccination effort. Deployment timelines
will be determined by the providing agency to ensure compliance with pre-deployment testing,
equipping, training and any other requirements identified. Detailed information regarding necessary
staffing and supplies by facility type can be found in Appendix D.
3.1.8. Information Management at the CVCs
Information management, for the purposes of this playbook, consists of three components: Data Collection
and Storage, Reporting, and Requests. “Data Collection” is any data needed to complete patient
registration, scheduling, or tracking/monitoring the vaccine doses. “Reporting” is any information that
would inform situational awareness or resource decision making, to include Essential Elements of
Information (EEI), outcomes, limiting factors, resource shortfalls, inventory stock, and processing delays.
The EEIs will be designated by the NRCC/RRCC in the form of an Information Collection Plan (ICP) that will
be socialized to ensure CVCs are aware of all necessary reporting requirements. “Requests,” primarily, are
top down Requests for Information (RFI). RFIs may include inquiries from internal or interagency partners.
Data Collection and Storage
The recommended best practice for information management at federally supported CVCs is to integrate
directly with STT processes for patient registration, scheduling, and other tasks requiring data collection
and storage for vaccine doses. This supports the President's directive of "federal support" to STT
vaccination campaigns by ensuring minimal disruption to existing processes. This unified approach also
maintains a singular and familiar process for the general public. To this end, a Privacy Threshold
Assessment has been authorized which allows federal employees and contractors to access and utilize
state information systems for the collection and storage of information (including PII/PHI) necessary to the
operation of a CVC. Each region must coordinate with their respective STT partners to procure
a Memorandum of Understanding (MOU) stating that all PII/PHI collected on federally supported CVCs will
only be entered and stored on STT run systems. Additionally, each MOU must state that all activities
necessary to the integration of federally supported CVCs with STT systems (such as training and granting
access to necessary employees) is solely the responsibility of the STT partner in question.
Reporting
Well-functioning reporting mechanisms adopt a bottom-up approach. Accurate and useful reporting is
typically communicated at the local level. Reporting, in the form of EEI, work assignments, outcomes,
shortfalls, and limiting factors will go through the CVCs, whenever practical. Reporting requirement such as
EEIs and Critical Information Requirements (CIRs) will be reported from the CVCs to the RRCC.
Requests
RFIs can be received from many different partners from within the federal government, from the media, or
from STT counterparts. Inquiries will be routed from either the NRCC or the RRCC to appropriate answering
parties. Inquiries and responses will be tracked utilizing WebEOC RFI tracking system.
3.2. Community Vaccination Center Operational Roles and Responsibilities
Clinic Manager
• In charge of clinical operations
• Coordinate overall clinical aspects of vaccine administration to recipients, ensuring quality control of vaccine
administration as well as proper storage and handling of vaccines, sharps and PPE use
Vaccinators

Page | 15
• Administer vaccination in accordance with EUA and STT requirements for IM administration
Registered Nurses
• Oversee the vaccinators if not vaccinating but also observe for 15 minutes or 30 minutes based on history for
anaphylaxis or side effects
• Can provide clinical information on questions from recipients
• Can also provide vaccine for recipients
• Should also monitor safety of the administration of vaccine by those not comfortable/have limited experience
with intramuscular injections
Clinic Flow; Reviewer
• Provides more detailed assessment and screening of recipients who “screen out” of the basic clinical
algorithm to receive the vaccine
Observation Area Manager
• Provides observation for adverse reactions in the observation area (could be performed by the RN)
Advanced Life Support Ambulances
• Observe recipients for adverse reactions to vaccine and provide general first aid for staff, volunteers and
recipients as needed; this needs medical oversight
• Must be ready for Advanced Cardiac Life Support requirements (not just epi pen).
Safety Officer
• Assures scene and worker safety; Monitor, investigate, and resolve or mitigate all safety considerations of
CVCs operations at the CVC. (May be a medical staff member or a non‐medical staff member)
• Provide oversight for personnel in attendance at the CVCs and staff ensuring protective measures, social
distancing, proper donning and doffing of PPE, and decontamination of actively touched surfaces, materials,
etc.
Medical Screeners
• Works alongside the registration area to assure that the candidates can proceed with vaccine administration,
address any medical questions
Vaccination Preparer
• Clinical staff to assist Pharmacist(s) readying vaccine for administration in accordance with EUA. Duties
include, but may not be limited to, transferring vials to vaccinators, drawing doses and preparing syringes
Pharmacists
• Optimally, would prepare doses of vaccine so that vaccinator can move the line better and get more vaccine
out
• Should have current unencumbered license
Pharmacy Techs
• Assist the pharmacist in high demand CVCs
• Works under authority of pharmacist
Forms (VIS/EUA) Distribution
• Provide initial greeting of public entering the CVCs and provide recipients with initial actions and directions to
stations within CVCs based on triage questions/protocol
General Staff
• Collect information; review pre-filled forms for accuracy, provide the VIS/EUA, collect consent; Who does the
registration for 2
nd
dose vaccine
Volunteer Coordinator
• Ensures staff (volunteer or paid) are accounted for, checked in to the CVC, assigned roles, oriented to the
facility, etc. (This role may be independent or performed by the Clinic Manager)
Check-In Staff
• Ensures sign‐in and out of all staff and volunteers assigned to the CVC, as well as supporting other critical
record‐keeping and documentation activities as assigned by the Clinic Manager. (May be performed by staff
who fill other roles during the CVC)
Administrative Staff
• Ensures sign-in and out of all staff and volunteers assigned to the CVC

Page | 16
• Supports other critical record-keeping and documentation activities as assigned by the Clinic Manager
Supply Manager
• Ensure that required vaccine and ancillary supplies are on CVC and are available in sufficient quantities
during CVC operations
• Supports or coordinates other logistical functions (food, cleaning service, etc.)
• Advises the Clinic Manager on issues related to equipment and supplies
• Works with Pharmacist and Clinic Manager to assure correct and sufficient doses of vaccine available,
sufficient CDC cards, additional documentation, required clinical supplies, and appropriate PPE
IT Support
• Work with CVCs staff to set up and maintain all information technology equipment required for CVCs
operations
Security Officers
• Monitor and have authority over internal and external security of CVC, personnel and operational equipment
and supplies, including pharmaceuticals
• Closely works with Safety Officer on hazard and safety issues or conditions
Traffic Control
• Keep people moving in the right direction
• Help recipients through the CVCs directing as needed to appropriate stations
• Ensuring recipients go to stations which are open and not busy, and maintain social distancing
Recipient Exit Area/Exit Reviewer
• Ensures all recipients receive all necessary educational forms about the incident and vaccine
• Answers basic questions about the vaccine and directs recipients to medical evaluation for complicated
questions
Language translation and ASL and language interpretation services
• Provide medical interpretation, usually via a contracted service or telephone line
External Affairs/Community Relations (on-call)
• Official spokesperson, approves all communication outside of the CVCs
Legal (on-call)
• Ensures that all federal tasks and activities at CVCs are in compliance with the law
• Provides high quality legal advice, counsel, risk analysis as the Point-of-Contact for the FEMA Office of Chief
Counsel
• Provides legal support to CVC federal leadership on all matters involving STT legal counsel
4.0 Administration
The RRCC is administratively responsible for all assigned resources, including overhead staff. The following are
general guidelines for common administrative tasks; deviations may occur for larger CVCs and require
concurrence from the Clinic Manager.
5.0 Oversight, Coordinating Instructions and Communications
5.1. Oversight
Oversight of the CVCs is conducted by the RRCC. The Regional Area Coordinators will liaise with the RRCC and
report to the Vaccination Task Force, who will inform the NRCC.
5.2. Coordinating Instructions
Coordinating vaccine administration and distribution across jurisdictions requires effective interagency
communication. In order to plan and scale vaccination programs, STT and must rely on both an advanced
understanding of their allocations and a timely delivery of their ordered doses. The program will be scaled
based on what is working best on the ground for state and local partners, and the communities they serve.
Appendix E describes the process to effectively address STT needs by providing Federal support to CVCs and
establishing CVCs.
5.3. Communications

Page | 17
All communications should follow the command and control procedures outlined in the Oversight section
above
Appendices
Appendix A: COVID-19 Community Vaccination Center Typing
Appendix B: Facility Type Conceptual Layouts
Appendix C: Civil Rights Considerations During COVID-19 Vaccine Distribution Efforts
Appendix D: Facility Type Force Packages by Positions and Equipment/Supplies
Appendix E: State to Federal Coordination Flowchart
Appendix F: Defining Federally Supported Sites
Appendix G: Critical Considerations for FEMA Employees

Page | 18
Appendix A: COVID-19 Community Vaccination Center Typing
COVID-19 Community Vaccination Center Types
FEMA, with Federal partners, has deployed tailorable packages to support states, tribes, and territories in the
establishment of community Vaccination Centers (CVCs). They are configured into five types below.
Type 1 Vaccination Clinic (Approximately 6,000 vaccinations/day capacity)
Federally supported site to include facility leasing, approximately 245 personnel (fixed site) or 269 (drive-through), equipment
and supplies to meet throughput over a 12-hour shift.
Facility
Clinical Force Package
Non-Clinical Force Package
1
Other Support
Minimum of 15,000 sf. with
adequate parking for at least 800
vehicles including accessible
services and parking
156 total clinical staff, including:
- 80 vaccinators
2
- 15 Registered Nurses
- 4 EMS personnel staffing two
ALS/Paramedic Ambulances
3
84-108 total non-clinical staff,
including:
- 5 command and control
- 20 law enforcement/security
- 5 IT support
Additional Supply Cache:
Gloves, masks, face shields
Computer and internet access,
Spare syringes, needles, alcohol
preps
Type 2 Vaccination Clinic (Approximately 3,000 vaccinations/day capacity)
Federally supported site to include facility leasing, approximately 159 personnel (fixed site) or 178 (drive-through), equipment
and supplies to meet throughput over a 12-hour shift.
Facility
Clinical Force Package
Non-Clinical Force Package
1
Other Support
Minimum of 7,500 sf. with
adequate parking for at least 600
vehicles including accessible
services and parking
95 total clinical staff including:
-
40 vaccinators
2
- 10 Registered Nurses
- 4 EMS personnel staffing two
ALS/Paramedic Ambulances
3
61-80 total non-clinical staff
including:
- 3 command and control
- 10 law enforcement/security
- 3 IT Support
Additional Supply Cache:
Gloves, masks, face shields
Computer and internet access,
Spare syringes, needles, alcohol
preps
Type 3 Vaccination Clinic (Approximately 1,000 vaccinations/day capacity)
Federally supported site to include facility leasing, approximately 87 personnel (fixed site) or 97 (drive-through), equipment
and supplies to meet throughput over a 12-hour shift.
Facility Clinical Force Package Non-Clinical Force Package
1
Other Support
Minimum of 4,500 sf. with
adequate parking for at least 250
vehicles including accessible
services and parking
54 total clinical staff including:
-
15 vaccinators
2
- 8 Registered Nurses
- 2 EMS personnel staffing one
ALS/Paramedic Ambulance
3
30-40 total non-clinical staff
including:
- 3 command and control
- 6 law enforcement/security
- 2 IT Support
Additional Supply Cache:
Gloves, masks, face shields
Computer and internet access,
Spare syringes, needles, alcohol
preps
Type 4 Vaccination Clinic (Approximately 250 vaccinations/day capacity)
Federally supported site to include facility leasing, approximately 43 personnel (fixed site) or 48 (drive-through), equipment
and supplies to meet throughput over a 12-hour shift.
Facility
Clinical Force Package
Non-Clinical Force Package
1
Other Support
Minimum of 2,500 sf. with
adequate parking for at least 130
vehicles including accessible
services and parking
26 total clinical staff including:
-
6 vaccinators
2
- 4 Registered Nurses
- 2 EMS personnel staffing one
ALS/Paramedic Ambulance
3
15-20 total non-clinical staff
including:
- 2 command and control
- 3 law enforcement/security
- 1 IT Support
Additional Supply Cache:
Gloves, masks, face shields
Computer and internet access,
Spare syringes, needles, alcohol
preps
Type 5 (Mobile) Vaccination Clinic (Approximately 250 vaccinations/day capacity)
Federally supported mobile site to include self-hauling capability, outdoor sheltered vaccination stations, approximately 49
personnel (fixed site) or 54 (drive-through), equipment and supplies to meet throughput over a 12-hour shift.
Facility Clinical Force Package Non-Clinical Force Package
1
Other Support
Minimum of 2,500 sf. of area to
set-up with adequate parking for
trucks and trailers plus support
staff and vaccine recipients
26 total clinical staff including:
-
6 vaccinators
2
- 4 Registered Nurses
- 2 EMS personnel staffing one
ALS/Paramedic Ambulance
3
21-26 total non-clinical staff
including:
- 2 command and control
- 3 law enforcement/security
- 1 IT Support
- 2 truck drivers (contract)
- 4 set-up/maintenance (contract)
Additional Supply Cache:
Same as Type 4 above.
Locally contracted requirements:
Toilets, generators, others as
required.
1
Legal, OER, ODIC, Civil Rights Advisors and other specialized support personnel will be on-call for all CVC but are not required to be on-site full-time.
External Affairs is projected to be on-site for Type 1 and Type 2 sites during vaccination operations.
2
Each STT must identify the personnel authorized by State Health law/regulation to administer intramuscular injections in their jurisdiction.
3
At least one Advanced Life Support (ALS) ambulance, staffed by a crew of two including at least one state certified/licensed paramedic will be on-site
during vaccination operations.
Page | 19
Appendix B: Facility Type Conceptual Layouts
Overview
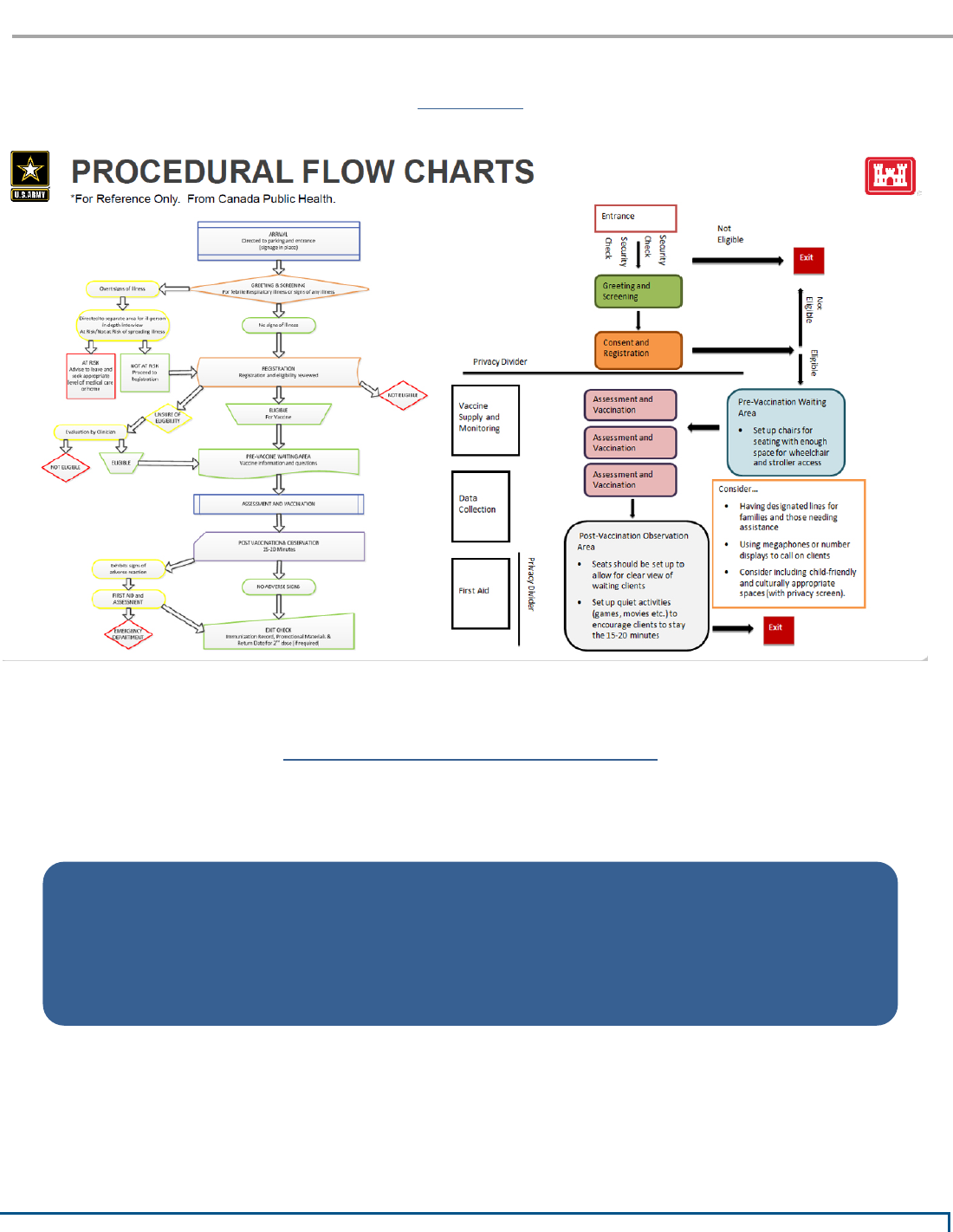
Fixed Facility (Walk-Through)
Gymnasiums, Schools, NBA/NFL Stadiums
• Facility size model goal = Type 3 (1,000 vaccinations a day)
• Type 2 (3,000) and Type 3 (1,000) facility size models can be replicated
side-by-side to increase throughput in existing larger facilities to create a
Type 1 model (6,000 vaccinations a day).

Page | 20

Page | 21

Page | 22
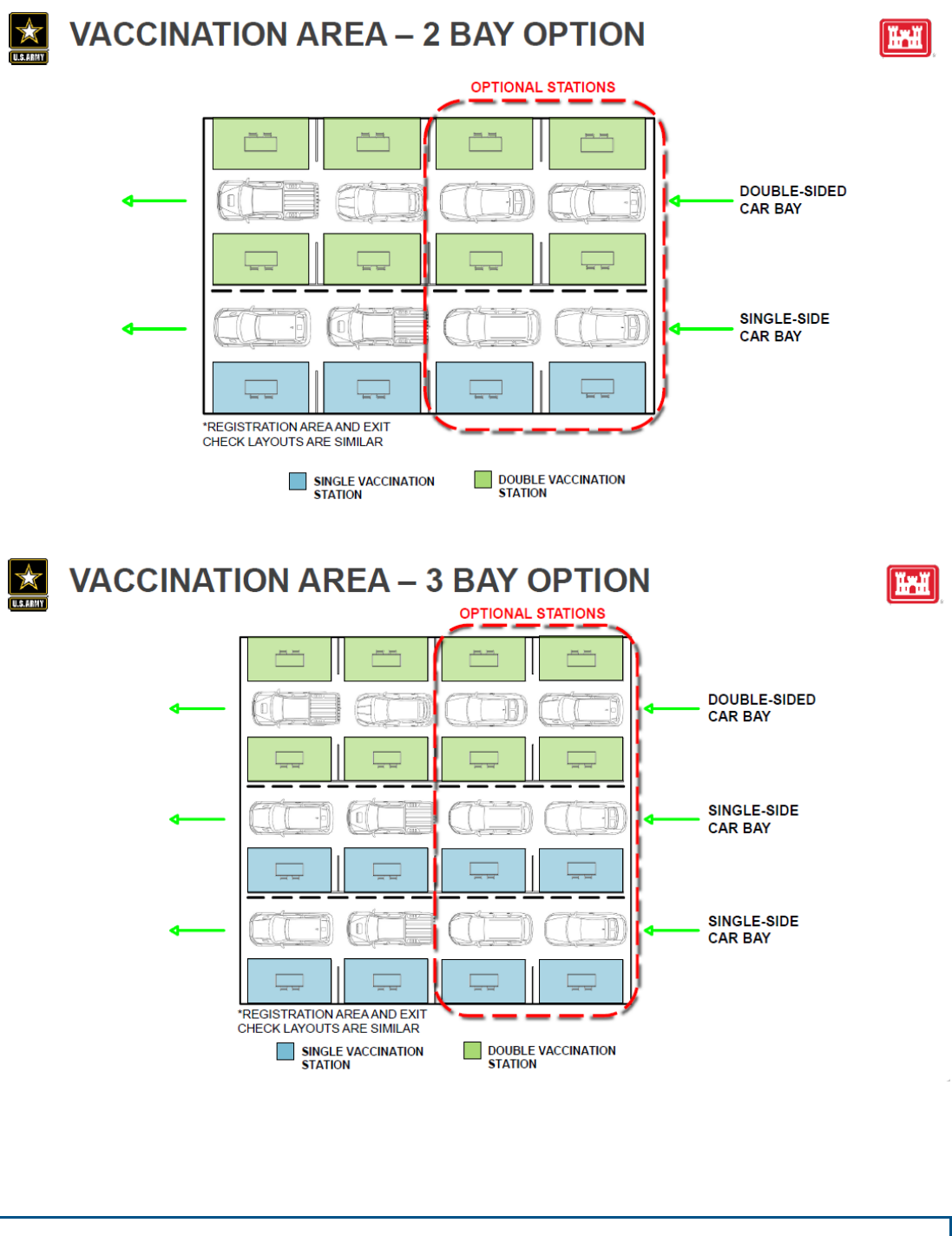
Drive-Through
Parking Lots at Big Box Stores, School/Colleges, Stadiums
Assumptions
• Coordinate traffic control, signage, barricades, and wayfinding with the local municipalities
and police departments.
• All facility sizes (Type 1 -4) may be accommodated by this model (based on size of available
flat lot).
• Lot is available for a 12-hour day (lights may be provided or installed as needed).
Size
• Type 1 (6,000 doses per 12-hour day) –538,000 SF flat lot (~12 acres)
• Type 2 (3,000 doses per 12-hour day) –270,000 SF flat lot (~6 acres)
• Type 3 (1,000 doses per 12-hour day) –90,000 SF flat lot (~2 acres)
• Type 4 (250 doses per 12-hour day) –23,000 SF flat lot (~.5 acre)
* For reference, a Wal-Mart Super Center parking lot can be up to 12 acres

Page | 23
Page | 24

Page | 25
Mobile Vaccination Clinic
Vaccination Trailer w/ attached
awning
Vaccination Support Trailer

FEMA Advisory
January 2021 1
Civil Rights Considerations During
COVID-19 Vaccine Distribution Efforts
To support FEMA’s efforts during the COVID-19 vaccine distribution efforts, FEMA’s Office of
Equal Rights (OER) provides this checklist for use by all partners to ensure access to
programs and activities and the impartial and fair provision of services.
Background
On March 13, 2020, the ongoing novel coronavirus (COVID-19) was declared a national emergency pursuant
to the Robert T. Stafford Disaster Relief and Emergency Act (Stafford Act). The COVID-19 pandemic, like all
emergencies, has affected people of different races and ethnicities, geographic area and income levels. The
Federal Emergency Management Agency (FEMA) is helping identify and fill resource gaps, using federal
funding to accelerate state vaccination efforts and working to establish vaccine sites, in alignment with the
President’s COVID-19 response plan.
FEMA remains committed to its mission of helping people before, during and after disasters by ensuring
access to its programs and services and enforcing civil rights. FEMA’s Office of Equal Rights is responsible
for ensuring compliance with and enforcement of FEMA’s external Civil Rights obligations under the Stafford
Act, Civil Rights Act, Rehabilitation Act, and Age Discrimination Act. FEMA also has responsibilities under
Executive Order 13166, Improving Access to Services for Persons with Limited English Proficiency, and
Executive Order 12898, Federal Actions to Address Environmental Justice in Minority Populations and Low-
Income Populations.
Civil Rights Considerations
Inclusive Planning
Item
Complete
Incomplete
Review community demographics data to identify:
1. Limited English proficient communities and languages for interpretation and
translation of critical vaccination information;
2. Communities unable to travel due to lack of public transportation or disabilities;
3. Communities without available or affordable internet access; and
4. Other underserved communities.
Appendix C: Civil Rights Considerations During COVID-19 Vaccine Distribution
Efforts

Civil Rights Considerations During COVID-19 Vaccine Distribution
Learn more at fema.gov January 2021
Develop plans to ensure equitable access to information and vaccination sites for all
communities, including underserved communities and those protected by law (i.e.,
race, color, national original, religion, sex, age, disability, English proficiency and
economic status).
Develop plans to conduct vaccinations for communities unable to travel, including the
use of accessible mobile units.
Develop messaging addressing concerns regarding site selection and accessibility,
underlying conditions, religious exemptions and safety concerns.
Develop process for citizens to file a complaint alleging a civil rights violation during
vaccinations and messaging regarding process.
Develop plans to increase public transportation, if necessary, for individuals to and
from vaccination sites.
Develop plans to support applicants in new virtual application processes, particularly
communities without available or affordable internet access.
Develop plans for the proper disposal of medical and other waste to ensure it does not
disproportionally affect any community.
Effective Communication Access
Item
Complete
Incomplete
Identify and conduct community engagement events with community-based and civil
rights organizations.
Conduct community engagement events with sign language interpreters and
captioning.
Conduct community engagement events in communities without reliable internet
adoption and/or access.
Include information on how to obtain accessible formats of documents on all
communications.
Ensure electronic information and information technology is accessible (i.e., Alt Text,
high contrast).

Civil Rights Considerations During COVID-19 Vaccine Distribution
Learn more at fema.gov January 2021
Ensure non-discrimination statement and contact for civil rights complaints on all
communication materials.
Increase communication access through social media platforms in ways that are
accessible to individuals with disabilities (i.e., Alt Text, Closed Captioned Videos).
Develop plans for individuals who are unable to wear masks due to medical or other
conditions or who require the removal of masks to communicate.
Language Access
Item
Complete
Incomplete
Translate vaccine and site information into commonly used languages in the
community, based on your review of community demographics.
Provide interpreters at community engagement events for commonly used languages.
Provide interpreters at vaccination sites or by telephone for commonly used
languages.
Include information on how to obtain translated documents on all communications.
Plan for the increased need for accessible and multilingual messaging and
communications through available ethnic media outlets, wireless emergency
communications, and use of virtual townhalls for coordinated communications.
Physical Accessibility
Item
Complete
Incomplete
Ensure meeting and vaccination sites are accessible by public transportation.
Ensure meeting and vaccination sites are compliant with ADA accessibility
requirements.
Document areas of noncompliance with ADA requirements and modifications made.
Ensure mobile vaccination units are accessible.
Ensure vaccination centers are equipped with assisted technology. (Ex: UbiDuos).

Civil Rights Considerations During COVID-19 Vaccine Distribution
Learn more at fema.gov January 2021
Pre-identify locations to account for the care of individuals requiring additional
assistance, including older adults, individuals with physical and cognitive disabilities
and others with access and functional needs.
Develop plans to provide reasonable accommodations, including persons who are
unable to wear a facemask due to a disability.
Ensure meeting and vaccination sites offer services to individuals with disabilities in
the most integrated setting appropriate.
Contact Us
If you have questions or would like assistance in completing any checklist item, please contact the
External Civil Rights Division within FEMA’s Office of Equal Rights. FEMA[email protected]
For copies of FEMA documents in alternative formats, please call 800-621-3362 (TTY: 800-462-7585).
If you speak a language other than English and need help with this document, please call 800-621-3362 (TTY:
800- 462-7585) and you will be connected to an interpreter who will assist you at no cost.
Si habla un idioma diferente al inglés y necesita ayuda con este documento, llame al 800-621-3362 (TTY: 800-
462- 7585) y lo contactaremos con un intérprete que lo ayudará sin costo alguno para usted.
Если вы не говорите на английском языке и нуждаетесь в помощи, позвоните по номеру 800-621-3362
(TTY: 800-462-7585). Вас соединят с переводчиком, который бесплатно поможет вам.
Se você fala um idioma além do inglês e precisa de ajuda em relação a este documento, ligue para 800-621-
3362 (TTY: 800-462-7585) e você será conectado a um intérprete que irá ajudá-lo sem nenhum custo adicional.
Nếu quý vị nói một ngôn ngữ khác Tiếng Anh và cần giúp đỡ với tài liệu này, hãy gọi 800-621-3362 (TTY: 800-
462- 7585) và quý vị sẽ được kết nối với một thông dịch viên, là người sẽ trợ giúp miễn phí cho quý vị.
영어를 사용하지 못하는 사람으로써 본 문서에 대해 도움이 필요할 경우, 전화 800-621-3362
(텔레타이프라이터: 800-462-7585)로 연락주시면 여러분을 무료로 도와줄 통역사와 연결해
드립니다.
Si vous parlez une langue autre que l’anglais et que vous avez besoin d’aide en rapport avec le présent
document, veuillez composer le 800-621-3362 (numéro TTY pour les malentendants : 800-462-7585) pour
qu’un interprète soit gratuitement mis à votre disposition.
Si w pale yon lang ki pa lang Angle e ou bezwen èd avèk dokiman sa a, tanpri rele 800-621-3362 (TTY: 800-
462- 7585) epi yo pral konekte w ak yon entèprèt ki pral ede w, gratis.
Civil Rights Considerations During COVID-19 Vaccine Distribution
Learn more at fema.gov January 2021
英語以外の言語でこのページの詳細をお知りになりたい方は、お電話で800-621-3362 (TTY: 800-462-7585)
までお問い合わせください。無料で通訳をご利用いただけます。
Kung nagsasalita ka ng wikang bukod sa Ingles at nangangailangan ng tulong sa dokumentong ito, mangyaring
tumawag sa 800-621-3362 (TTY: 800-462-7585) at maikokonekta ka sa isang interpreter (tagasalin sa wika)
na tutulong sa iyo nang walang bayad.
如果您使用除英语之外的其他语言并且就本文件需要帮助,请致电800-621-3362(听障及语障用户(TTY):
800-462-7585),您将与翻译人员联系,该翻译人员将为您提供免费帮助。
ﻋﻦ ﺔﻋﺎﺒﻄﻟا) 3362-621-800 ﺑﺎﻟﺮﻗﻢ لﺎﺼﺗﻻا ﯾﺮﺟﻰ ،ﺔﻘﯿﺛﻮﻟا ﺗﻠﻚ ﻣﻊ ةﺪﻋﺎﺴﻣ ﻰﻟإ ﺖﺠﺘﺣاو ﺔﯾﺰﯿﻠﻜﻧﻹا ﻏﯿﺮ ﻟﻐﺔ ﺗﺘﻜﻠﻢ ﻛﻨﺖ اذإ
ﻣﺠﺎﻧﺎ ةﺪﻋﺎﺴﻤﻟا ﻟﻚ مﺪﻘﯿﺳ ﺷﻔﮭﻲ ﻣﺘﺮﺟﻢ ﻣﻊ ﻚﻠﺻو ﻢﺘﯿﺳو 7585-462-800( :ﺑﻌﺪ.

Page | 32
Appendix D: Facility Type Force Packages by Positions and Equipment/Supplies
Type 1 - 6,000 doses a day
Facility Dimensions
• Minimum of 15,000 sq. ft
• Site Command and Control (5): Team Lead and Deputy (2), Clinical Coordinator (1), Operations Section Chief
(1), Logistics Section Chief (1)
• Total Personnel: 245 fixed site / 269 drive-through (156 clinical, 84 non-clinical [108 drive-through], 5 C2)
Equipment and Supplies
Medical Supplies
Qty
General Supplies
Qty
IT Supplies
Qty
Gloves
TBD
Data entry forms
TBD
Laptops
50
Epi-Pens
12
Tables
TBD
Internet Connectivity
Yes
First Aid Kits
4
Chairs
TBD
iPad
100
Face Shields
100
Dollies
3
Chargers
TBD
N-95 Respirators
100/day
Storage Equipment
TBD
Electric Generators (if necessary)
TBD
Alcohol Swabs
10000/day
Refrigerators & Freezers
TBD
Handheld Land-Mobile Radios
70
Syringes
8000/day
Bathroom facilities
Yes
Vaccination Record Cards
6100/day
Signage
TBD
Drive-Through Requirements
Variable message signs
TBD
Traffic Cones
500
Tents/Shelter
TBD
Clinical Staff
Position
Per
site
Vaccinators
80
Vaccine Preparers 20
Pharmacist
1
Pharmacy Techs
5
Medical Screeners
20
Clinic Flow; Reviewer
5
Recovery Area Manager
3
Clinic Manager 2
Patient Exit Area/Exit Review
1
RN
15
Advanced Life Support Ambulances (two
ambulances with crew of 2 each)
4
Non-Clinical Staff
Position
Per
site
Security
20
Traffic Control* (*drive through sites require
more traffic control personnel – site
dependent)
10
*+20
Safety* (*drive through sites require more
safety personnel – site dependent)
2
*+4
Supply Manager
2
IT Support
5
Forms (VIS) Distribution staff
1
Orientation/Information
2
Language translation and ASL and language
interpretation services
TBD
General Staff
20
External Affairs/Community Relations
1
Administrative Staff
20
Volunteer Coordinator
1

Page | 33
Type 2 - 3,000 doses a day
Facility Dimensions
• Minimum of 7,500 sq. ft
• Site Command and Control (3): Team Lead and Deputy (2), Clinical Coordinator (1)
• Total Personnel: 159 fixed site / 178 drive-through (95 clinical, 61 non-clinical [80 drive-through], 3 C2)
Equipment and Supplies
Medical Supplies
Qty
General Supplies
Qty
IT Supplies
Qty
Gloves
TBD
Data entry forms
TBD
Laptops
50
Epi-Pens
12
Tables
TBD
Internet Connectivity
Yes
First Aid Kits
4
Chairs
TBD
iPad
100
Face Shields
100
Dollies
3
Chargers
TBD
N-95 Respirators
100/day
Storage Equipment
TBD
Electric Generators (if necessary)
TBD
Alcohol Swabs
10000/day
Refrigerators & Freezers
TBD
Handheld Land-Mobile Radios
50
Syringes
6000/day
Bathroom facilities
Yes
Vaccination Record Cards
3100/day
Signage
TBD
Drive-Through Requirements
Variable message signs
TBD
Traffic Cones
400
Tents/Shelter
TBD
Clinical Staff
Position
Per
site
Vaccinators 40
Vaccine Preparers
10
Pharmacist
1
Pharmacy Techs
3
Medical Screeners
15
Clinic Flow; Reviewer
6
Recovery Area Manager 1
Clinic Manager
2
Patient Exit Area/Exit Review
3
RN
10
Advanced Life Support Ambulances (two
ambulances with crew of 2 each)
4
Non-Clinical Staff
Position
Per
site
Security
10
Traffic Control* (*drive through sites require
more traffic control personnel – site
dependent)
8
*+16
Safety* (*drive through sites require more
safety personnel – site dependent)
2
*+3
Supply Manager
2
IT Support
3
Forms (VIS) Distribution staff
2
Orientation/Information
2
Language translation, ASL and language
interpretation services
TBD
General Staff
20
External Affairs/Community Relations
1
Administrative Staff
10
Volunteer Coordinator
1

Page | 34
Type 3 - 1,000 doses a day
Facility Dimensions
• Minimum of 4,500 sq. ft
• Site Command and Control (3): Team Lead and Deputy (2), Clinical Coordinator (1)
• Total Personnel: 87 fixed site / 97 drive-through (54 clinical, 30 non-clinical [40 at drive-through], 3 C2)
Clinical Staff
Position
Per
site
Vaccinators 15
Vaccine Preparers
6
Pharmacist
1
Pharmacy Techs
3
Medical Screeners
10
Clinic Flow; Reviewer
6
Recovery Area Manager 1
Clinic Manager
1
Patient Exit Area/Exit Review
1
RN
8
1 Advanced Life Support Ambulance (crew of
two)
2
Non-Clinical Staff
Position
Per
site
Security
6
Traffic Control* (*drive through sites require
more traffic control personnel – site
dependent)
4
*+8
Safety* (*drive through sites require more
safety personnel – site dependent)
1
*+2
Supply Manager
2
IT Support
2
Forms (VIS) Distribution staff
1
Orientation/Information
2
Language translation. ASL and language
interpretation services
TBD
General Staff
5
External Affairs/Community Relations
1
Administrative Staff
5
Volunteer Coordinator
1
Equipment and Supplies
Medical Supplies
Qty
General Supplies
Qty
IT Supplies
Qty
Gloves
TBD
Data entry forms
TBD
Laptops
50
Epi-Pens
6
Tables
TBD
Internet Connectivity
Yes
First Aid Kits
2
Chairs
TBD
iPad
100
Face Shields
20
Dollies
3
Chargers
TBD
N-95 Respirators
30/day
Storage Equipment
TBD
Electric Generators (if necessary)
TBD
Alcohol Swabs
3000/day
Refrigerators & Freezers
TBD
Handheld Land-Mobile Radios
30
Syringes
2000/day
Bathroom facilities
Yes
Vaccination Record Cards
1100/day
Signage
TBD
Drive-Through Requirements
Variable message signs
TBD
Traffic Cones
200
Tents/Shelter
TBD

Page | 35
Type 4 - 250 doses a day
Facility Dimensions
• Minimum of 2,500 sq. ft
• Site Command and Control (2): Team Lead and Deputy (2)
• Total Personnel: 43 fixed site / 48 drive-through (26 clinical, 15 non-clinical [20 at drive-through], 2 C2)
Equipment and Supplies
Medical Supplies
Qty
General Supplies
Qty
IT Supplies
Qty
Gloves
TBD
Data entry forms
TBD
Laptops
6
Epi-Pens
6
Tables
TBD
Internet Connectivity
Yes
First Aid Kits
2
Chairs
TBD
iPad
12
Face Shields
10
Dollies
0
Chargers
TBD
N-95 Respirators
12/day
Storage Equipment
TBD
Electric Generators (if necessary)
TBD
Alcohol Swabs
1000/day
Refrigerators & Freezers
TBD
Handheld Land-Mobile Radios
12
Syringes
500/day
Bathroom facilities
Yes
Vaccination Record Cards
300/day
Signage
TBD
Drive-Through Requirements
Variable message signs
TBD
Traffic Cones
150
Tents/Shelter
TBD
Clinical Staff
Position
Per
site
Vaccinators 6
Vaccine Preparers
3
Pharmacist
1
Pharmacy Techs
1
Medical Screeners
5
Clinic Flow; Reviewer
1
Recovery Area Manager 1
Clinic Manager
1
Patient Exit Area/Exit Review
1
RN
4
1 Advanced Life Support Ambulance (crew of
two)
2
Non-Clinical Staff
Position
Per
site
Security
3
Traffic Control* (*drive through sites require
more traffic control personnel – site
dependent)
2
*+4
Safety* (*drive through sites require more
safety personnel – site dependent)
1
*+1
Supply Manager
1
IT Support
1
Forms (VIS) Distribution staff
1
Orientation/Information
1
Language translation, ASL and language
interpretation services
1
General Staff
2
Administrative Staff
2

Page | 36
Type 5 (Mobile) - 250 doses a day
Site Area Dimensions
• Minimum of 2,500 sq. ft of unobstructed, paved area
• Site Command and Control: Team Lead and Deputy (2)
• Total Personnel: 49 fixed site / 54 drive-through (26 clinical, 21 non-clinical [26 at drive-through], 2 C2)
Clinical Staff
Position
Per
site
Vaccinators 6
Vaccine Preparers
3
Pharmacist
1
Pharmacy Techs
1
Medical Screeners
5
Clinic Flow; Reviewer
1
Recovery Area Manager 1
Clinic Manager
1
Patient Exit Area/Exit Review
1
RN
4
1 Advanced Life Support Ambulance (crew of
two)
2
Non-Clinical Staff
Position
Per
site
Security
4
Traffic Control* (*drive through sites require
more traffic control personnel – site
dependent)
2
*+2
Safety* (*drive through sites require more
safety personnel – site dependent)
1
*+1
Supply Manager
1
IT Support
1
Forms (VIS) Distribution staff
1
Orientation/Information
1
Language translation, ASL and language
interpretation services
TBD
General Staff
2
Administrative Staff
2
Truck Drivers (contract)
2
Setup/Maintenance (contract)
4
Equipment and Supplies
Medical Supplies
Qty
General Supplies
Qty
IT Supplies
Qty
Gloves
TBD
Data entry forms
TBD
Laptops
6
Epi-Pens
6
Tables
TBD
Internet Connectivity
Yes
First Aid Kits
2
Chairs
TBD
iPad
12
Face Shields
10
Dollies
0
Chargers
TBD
N-95 Respirators
12/day
Storage Equipment
TBD
Electric Generators (if necessary)
TBD
Alcohol Swabs
1000/day
Refrigerators & Freezers
TBD
Handheld Land-Mobile Radios
12
Syringes
500/day
Bathroom facilities
Yes
Vaccination Record Cards
300/day
Signage
TBD
Drive-Through Requirements
Variable message signs
TBD
Traffic Cones
150
Tents/Shelter
TBD

Page | 37
Appendix E: State to Federal Coordination Flowchart
This chart describes the process to effectively address STT needs by providing Federal Support to CVCs and
Establishing CVCs.
States, Tribes, and
Territories (STT)
(CVCs)
Send Request to NRCC
for Processing
Submit Resource
Request Form (RRF)
Process and Fulfill
Requests
• Determine vaccination
capability/gaps that may
require federal
assistance
• Identify resource
needs/requirements to
establish additional
facilities and
mobile/drive-through
CVCs
• Determine requirement
for federally supported
vaccination
augmentation staffing
capability (i.e. FEMA’s
incident workforce)
•
If the request is for a
regional asset, the RRCC
will process it by acquiring
the resource or service
• If the request is for a
National asset, the RRCC
will send the request to
the NRCC for processing
and fulfillment
FEMA Regional Response
Coordination Center
(RRCC)
FEMA National Response
Coordination Center
(NRCC)
• Process RRFs from the
RRCC and delegate
resources to the regions,
once adjudicated. (Clinical
resources will be
adjudicated through the
NRCC Vaccine Task Force,
Non-Clinical resources will
be adjudicated by the
FOD Adjudication Cell)
• Coordinate federal
resources to meet STT
requirements
• Establish and
disseminate unified
guidance regarding
federally
supported/federally
operated community
vaccination centers.

Page | 38
Appendix F: Defining Federally Supported Sites
FEMA is providing a range of support to state, local, tribal, and territorial governments to assist, augment, and
expedite delivery of COVID-19 vaccinations in the United States.
FEMA is providing funding, personnel, and other resources through a variety of mechanisms to support
vaccination efforts. In considering sites that contribute to the President’s goal of 100 federally supported
sites in 30 days following Inauguration, FEMA will consider the following parameters:
What does it mean for a vaccination site to be “Federally supported”?
Federal support to vaccination sites could include some combination of:
Personnel
o Includes federal deployment of personnel or contractors, either in clinical or non-clinical roles
o National Guard under Title 32 orders will be considered as federal support where orders were
modified after January 20, 2021 resulting in an increased number of personnel supporting the site
(not just a change to the cost share for existing personnel). T-32 orders for
vaccination support that were issued before January 20th and not modified after that date will not
count toward the goal of 100 sites in 100 days
Materiel
o Includes tangible personal property, such as durable medical equipment or consumable supplies,
mobile vaccination capabilities, and/or real property provided by the federal government, other
than vaccine or vaccine kits
Funding
o Includes funding for materiel, facilities, staffing, etc. to be used at the vaccine site
o Includes project worksheets directly contributing to an operational vaccination site. Regions
should work closely with their states to confirm when these PA-supported sites become
operational and obtain specific location information (address and throughput estimates).
o Does not include the cost of vaccines and/or ancillary kits for vaccination
o Note: Funding from multiple federal agencies to the same site will be counted as one site
A Federally
Supported
site exists when…
1. A state established vaccination site has one or more of the following…
Federal Personnel
Federal Materiel
Federal Funding
2. The federal support enables the site to open, remain open, or expand capacity (Note: sites that close
between doses 1 and 2, and mobile sites are counted as 1 federally supported site)
3. AND when the site is, or has been, operational (meaning it is or has been open and actively accepting
persons for vaccination), on or after January 20, 2021. This is because we are seeking to expand
existing capacity.*
*Any site exclusively providing ancillary/support services such as a call center or logistics warehouse, unless co-located with a site providing
vaccinations, will not be included in this count.

Page | 39
Appendix G: Critical Considerations for FEMA Employees
FEMA is responsible for ensuring that Incident Management Work Force (IMW) personnel, including members of the
Surge Capacity Force, are paid whatever overtime they are entitled to under the law, and for avoiding over- or
under-payments. Under the Fair Labor Standards Act (FLSA), OCCHCO may decide that deployments to support
COVID-19 vaccinations are “emergencies.” An emergency designation may change whether work done by deployed
personnel is covered under the FLSA and qualifies for the payment of overtime. When the FLSA’s emergency
provisions apply –
1. FLSA non-exempt (FLSA covered) employees remain non-exempt while deployed, no matter whether they
are assigned to FLSA exempt or non-exempt work. Their overtime is paid at time and a half, and does not
count toward the bi-weekly and annual pay caps.
2. When FLSA-exempt (FLSA non-covered) employees deploy, their duties may change significantly from the
duties they perform in their steady-state position.
a. If deployed FLSA-non-covered employees do 51% or more FLSA covered work during a 7-day
period, their work for the entire week is covered by the FLSA. Their overtime is paid at the time
and a half rate and does not count toward the biweekly and annual pay caps. Each 7 day
period’s work must be evaluated to determine whether the FLSA non-covered employee spent
51% or more of his/her time performing FLSA covered work.
b. If deployed FLSA-non-covered employees do mostly FLSA-non-covered work while deployed,
they are generally paid at their hourly rate for each hour of overtime. Their overtime counts
toward the bi-weekly and annual pay caps.
Field leaders are strongly encouraged to assign FLSA non-covered employees to duties that are either
covered or not covered under the FLSA when they begin their deployments. Field leaders may reassign
them from FLSA covered to non-covered work (or vice versa), but if they do, they must clearly communicate
any changes to the deployed employee’s timekeeper.
The following types of work will be presumed to be covered under the FLSA:
• Greeter: These employees will welcome visitors to the site and direct any visitors to where they
would need to go to at the site based on the purpose of the visit.
• Administrative Support Specialist: These personnel will perform administrative duties, such as
visitor check in, collecting and filing documentation, data management, and non-IM planning
activities. They will not carry out patient administration duties.
• General Support Specialists: These personnel will provide logistical assistance, as well as other
administrative, facility, and operational support.
• Guides: These personnel will help direct visitors, ensure compliance with all social distancing rules
in the designated areas of the building/property, and manage the movement of persons and
vehicles within the site.
The following types of work will be presumed
not
to be covered under the FLSA:
• the supervision of other personnel;
• the obligation or commitment of more than $10,000;
• the regular exercise of discretion or independent judgment on matters of significance;
• making recommendations with regard to management, business operations, or the evaluation of
courses of action.
• the design or engineering of information technology systems or software (but may include the
issuance, maintenance, or repair of electronic devices or equipment);
• work such as the practice of medicine, law, nursing, or engineering that requires an advanced
degree or professional licensing or credentialing.

Page | 40
Acronyms List
ALS
Advanced Life Support
ASL
American Sign Language
ASPR
Office of the Assistant Secretary for Preparedness and
Response
BIA
Bureau of Indian Affairs
CDC
Centers for Disease Control and Prevention
CIR
Critical Information Requirement
COOP
Continuity of Operations
CVC
Community Vaccination Center
EEI
Essential Element of Information
ESF
Emergency Support Function
EUA
Emergency Use Authorization
FEMA
Federal Emergency Management Agency
GSA
General Services Administration
HHS
United States Department of Health and Human Services
ICP
Information Collection Plan
IHS
Indian Health Service
IIS
Immunization Information System
IM
Incident Management
IS
Incident Support
LUA
License and space Utilization Agreement
MA
Mission Assignment
MOU
Memorandum of Understanding
NRCC
National Response Coordination Center
PHI
Public Health Information
PII
Personal Identifiable Information
PPE
Personal Protective Equipment
PSPS
Public Safety Power Shutoff
PTA
Privacy Threshold Assessment
RFI
Request for Information
RRCC
Regional Response Coordination Center
RRF
Resource Request Form
STT
State, Tribal, Territorial
VAMS
Vaccine Administration Management System
VTrckS
Vaccine Tracking System

Page | 41
Glossary
Awardees – This is the term used in VTrckS to describe participating state, local, and territorial health
departments.
Appointment – The defined date and time a recipient was given by the STT to show up to a CVC site and
receive their vaccine.
Check-In/Screening Area – The area of a CVC site staffed by the where recipients arrive, are checked in and
where verification happens that they have an appointment. This area is also where any documents are
handed out to recipients.
COOP – The Continuity of Operation Plan is the site specific plan that addresses contingencies that may
impact the regular functioning of the CVC site and includes how to ensure continuous electrical power to the
cold storage freezers and also the how to rapidly close and relocate a CVC site in the event of severe weather
or other impacts that will disrupt site operations.
Community Vaccination Center (CVC)– A CVC are the location used to deliver vaccines. The CVC site refers to
the structure and parking spaces adjacent to the structure that are managed while the CVC site is in
operation.
Daily Shift/Safety Briefing – The meeting conducted with all CVC staff at the beginning of each day to review
relevant information and updates. This briefing is conducted each day prior to the CVC site opening.
Demobilization/Transition Plan – This plan is developed to organize the demobilization of the site and either
close the site and or to transition the site to be managed by non-federal agency (the state, local jurisdiction, or
tribal territory, etc.). This plan will address the closeout of contracts and relocation of all federal equipment.
Drive-Through CVC– A vaccination site in which the recipients do not exit their vehicle to enter a structure and
will stay in their car or next to their car the entire time.
Essential Elements of Information – The FEMA Headquarters defined information that CVC sites will report to
higher authority as defined.
FEMA Disaster Facility Setup Guide – The FEMA guide that establishes national guidance on the best
practices to lease and setup disaster facilities. This This Guide has been developed to ensure consistent and
clear guidance to facilitate timely and successful response and recovery operations. This Guide is not
designed to be prescriptive; emergency management requires flexibility to adapt to the incident and state
priorities.
First-Aid Station – The designed area at a CVC site where recipients would be handed off and received by
locally sources ambulance to handle any medical problems while they are at the CVC site. This area is not
staffed by federal personnel.
Fixed Facility CVC Site – Any facility or structure that is used for the distribution of vaccines.
Immunization Information System – Any state managed information system that is used to track the
vaccination process. These systems will vary across the STTs and CVC site staff will need some training to be
familiarized with the system.
Information Collection Plan – The plan that describes the overall process to collect, store, and transmit
information collected during the operation of the CVC site.
Intake Form – The document used to collect information from the recipient upon their arrival at the Check-
In/Screening Area.

Page | 42
License and Space Utilization Agreement – The legal agreement between the federal government and the
owner of the site that outlines the conditions of using the space while the CVC site is in operation.
Manufacturer Vaccine Handling Process – The manufacturers defined process to properly handle the vaccine
during the shipment, on-site storage, removal from cold-storage and preparation of the vaccine to be given to
a recipient. Each manufacturer will publish a specific vaccine handling process for their product.
Medical Screener – The CVC staff responsible to interview the recipient to identify any contraindications,
determine any precautions or pre-existing conditions. These questions may be accomplished using a locally
developed questionnaire.
Mobile Vaccination Clinic– A mobile vaccination site the able to independently move to different locations and
has a self-hauling capability, all-weather tentage, and is staffed with approximately 49 personnel.
Observation Area – This is also referred to as the Post Waiting Area and is the space for recipients to wait for
15 to 30 minutes after receiving their vaccine to ensure they do not have a negative reaction to the dose. The
vaccine recipient leaves this area and exit the facility once the observation time is over.
On-Site Security – The law enforcement personnel responsible for the overall security of the CVC site to
include responsibly to handle disruptive recipients or protesters at the CVC site.
Personnel Identifying Information (PII) – Information that if lost, compromised, or disclosed without
authorization, could result in substantial harm, embarrassment, inconvenience, or unfairness to an individual.
Examples of PII include: social security number, or biometric identifier (e.g., fingerprint, iris scan). Other data
elements such as a driver’s license number, financial information, citizenship or immigration status, or
medical information, in conjunction with the identity of an individual, are also considered sensitive PII.
PPE Allocation – The quantity of personnel protective equipment (PPE) that a CVC site will be given and
consists of two pieces of information – the total quantity of each type of PPE, and the date it will arrive at the
CVC site. This information will be used to inform PPE burn rate calculations. NOTE: The PPE allocation is
determined by the NRCC, which determines both the quantity and delivery date of any PPE allocation to any
CVC site.
Receiving Jurisdiction – The government agency that has jurisdictional authority where the CVC site is located.
Coordination will occur between the CVC site and the receiving jurisdiction to discuss delivery details.
Recipient Exit Area – The area of a CVC site where recipients leave the site.
State Tribal and Territories (STT)– These are the three government entities that can request a CVC site. Local
jurisdictions (cities or counties) are not included on this list and any requests for a CVC site is to be routed
through their State EOC to be forwarded to the RRCC.
Standby Ambulance – An ambulance that has been sourced locally to provide care to recipients in need of
medical and potential transportation off site to a more definitive care facility.
Staffing Plan – The schedule for personnel to continuously staff each position in the CVC site for the day to
include times for staff breaks and meals.
Traffic/Access Control Plan – The detailed plan that describes the access control procedures to ensure entry
and exit to the CVC site is managed. For a drive-through CVC site, the plan will describe the pathway that
vehicles will travel at the site and the safety procedures that CVC staff will follow when working Drive-through
Sites. The plan may also be developed to manage the arrival of vehicles and public transportation at Fixed
and Mobile CVC sites as well.
Training Plan – The list of training to be completed by staff working at the CVC site. The training plan is
developed at the CVC site and will be specific to the site and specific to certain positions. The intent of the
training plan is to describe the topics that staff need to understand prior to assuming their position. Training

Page | 43
topics include any specific STT or local training requirements, how to complete documents, reporting
requirements, how to use any websites. The training may be provided in a variety of ways, to include Just-In-
Time Training, webinars, or individual briefings.
Vaccinator – A person that meets the requirements of the STT to be eligible to administer the vaccine dose to
a recipient in accordance with guidance and recommendations.
Vaccination Site Assessment – The survey conducted by the key participants to determine the suitability of the
site to serve as a CVC. The key participants in this survey are Local Public Health Officials, Safety, Security,
Civil Rights, Emergency Management Officials, Fire Inspector & Office of Disability Integration Coordination.
Vaccination Station – The area where a recipient will physically receive their dose from the vaccinator.
Vaccine Allocation – The amount of vaccine doses that a CVC site is to be given and consists of two pieces of
information – the total number of doses and the date it will arrive at the CVC site and be considered eligible to
administer to a recipient. NOTE: The STT is always the agency that determines both the quantity and delivery
date of any vaccine allocation to any CVC site.
Vaccine Inventory – The total number of vaccine doses at the CVC site and the end of the day and once the
CVC site has completed vaccinations for the day. This number will be included in the Essential Elements of
Information reported at the end of shift.
Vaccine Recipient – A person that has been designated by the STT to receive a vaccine dose and has arrived
at the CVC site on the day of their appointment.
Vaccine Tracking System (VTrckS) – A secure, web-based information technology system managed by the CDC
that integrates the entire publicly-funded vaccine supply chain from purchasing and ordering through
distribution to participating state, local, and territorial health departments (referred to as ‘awardees’) and
health care providers.
