
Going Beyond Drug Seizures
Cocaine: A spectrum of products

2
e cocaine market presents a clear threat at global level.
Well-defined locations of production in South America and
large consumer markets in the Americas and Europe lead to
trafficking routes from a circumscribed origin to specific,
even if far-flung, destinations. While some parts of the world
play a crucial role as transit regions, the routes, modali-
ties and networks employed by criminal actors continue to
evolve, diversify and become more efficient. e increasingly
globalized, interconnected, digitalized and technologically
sophisticated nature of society, as well as a growing affluent
demographic in some regions where cocaine use has tradi-
tionally been low, can potentially catalyse and accelerate the
dynamism and expansion of the market.
e series Cocaine Insights, developed by UNODC in the
framework of the CRIMJUST programme and in coopera-
tion with partners and stakeholders at national, regional
and international levels, delivers the latest knowledge and
trends on issues related to cocaine markets in an accessible
and informative format.
Suggested citation: UNODC, Cocaine – a spectrum of products,
Cocaine Insights 2, UNODC, Vienna, October 2021.
Acknowledgements
Main contributors: Chloé Carpentier, Laurent Laniel,
Antoine Vella,Yulia Vorobyeva.
e authors are especially grateful to the SIMCI team in
the UNODC Country Office in Colombia, in particular
Mr Hernando Bernal and his team, for their support in
developing the report and for their substantive input.
is issue also benefited from invaluable advice from other
UNODC colleagues in the Research and Trend Analysis
Branch, the Laboratory and Scientific Service, the Country
Office in Bolivia and the Country Office in Peru.
is issue was produced thanks to the financial contribution
of the European Union.
Disclaimer
is publication has not been formally edited. e content
of this publication does not necessarily reflect the views or
policies of UNODC or any contributory organization, nor
does it imply any endorsement.
Comments on the report are welcome and can be sent to:
Drug Research Section
Research and Trends Analysis Branch
United Nations Office on Drugs and Crime
PO Box 500
1400 Vienna, Austria
Cocaine Insights
Table of Contents
Executive summary
Policy implications
The cocaine production process
Introduction
References
Consumer products
COCAINE: A SPECTRUM OF PRODUCTS
7
38
3
5
6
12
DEA
Drug Enforcement
Administration (United States)
EMCDDA
European Monitoring Centre for
Drugs and Drug Addiction
SIMCI
Sistema Integrado de Monitoreo
de Cultivos Ilícitos
UNODC
United Nations Office on Drugs
and Crime
Abbreviations
COCAINE INSIGHTS

3
In the context of an ongoing expansion of the global
cocaine market, this report summarizes the current state
of knowledge on what the cocaine consumer products are
and how they are produced and consumed in different
world regions. e report is based on available published
evidence and on the knowledge gained through UNODC’s
monitoring activities in South American countries.
It offers insight into the spectrum of cocaine products
in order to assist practitioners in drug supply and drug
demand reduction, such as law enforcement agencies and
healthcare providers, to tailor their response to production,
trafficking and consumption of cocaine products.
Knowledge gaps still remain in many world markets regard-
ing the cocaine products available to users, in terms of their
chemical form, purity, cutting agents used for dilution and
adulteration, price and routes of administration.
Cocaine is consumed worldwide in
a base or a salt form
Cocaine, an alkaloid extracted from the leaves of two species
of coca plant, is found worldwide in a variety of consumer
products that come in two chemical forms, as a base and
as hydrochloride salt. Nasal insufflation (“sniffing”, “snort-
ing”) of cocaine in its salt form, and the inhalation of the
vapours when cocaine in its base form is smoked, are the
most frequently used routes of administration at global
level, followed by injection and oral use.
Depending on the main ingredient and the method of
manufacturing, it is possible to distinguish three main
families of products derived from the base and salt forms:
(1) manufacturing process consumer products (MCPs)
derived from coca paste and cocaine base;
(2) freebase consumer products (FCPs) derived by convert-
ing cocaine salt back to base form;
(3) consumer products based on cocaine hydrochloride
(typically in powder form).
Manufacturing process consumer
products (MCPs) popular in South
America under different street
names
MCPs, mostly smokable substances made from coca paste
and/or cocaine base, are mainly found in South America.
Street names for these products vary from country to
country. Moreover, one name can refer to different prod-
ucts in different countries. A range of MCPs, most likely
made from coca paste are referred to as paco (Argentina,
Executive summary
Uruguay), pitillo (Bolivia), merla (Brazil), mono (Chile),
basuco (Colombia and Venezuela, where it is sometimes
adulterated with caffeine and phenacetin), baserolo (Ecua-
dor), pay (Peru), chespi (Paraguay) among others. ese
products are typically smoked, both mixed with tobacco
or marijuana, or pure using home-made pipes.
Some of the MCPs made of a solid form of cocaine base
found on South American markets are referred to as “crack”
by local consumers. However, this should be differentiated
from the conventional term “crack” that refers to a product
obtained from cocaine hydrochloride available in North
American and European markets.
“South American Crack”
“Crack”, a solid form of cocaine base, is especially popu-
lar in Brazil, while its variants are also found in Uruguay,
Paraguay as well as in coca producing countries (Bolivia,
Colombia and Peru). While products known as “crack” in
South America may come in multiple forms, they typically
differ from those found in North America and Europe,
in that they appear to be predominantly obtained from
cocaine in base form.
e so-called “South American crack” made in Bolivia,
Colombia and Peru may be smuggled to other South
American countries where it is received in 1-kilo bricks
and retailed in the form of small rocks, but it is also pos-
sible that “crack” is manufactured in destination countries
such as Brazil from dried coca paste and/or cocaine base
trafficked from Andean countries and pressed into 1-kilo
bricks.
Freebase and “crack” can both be
obtained from cocaine hydrochlo-
ride, but “crack” is less pure, easier
to make and more prevalent
e freebase consumer products (FCP) include “freebase”
in addition to “crack” as found in the European and North
American markets. Both of these primarily smokable prod-
ucts are prepared by transforming the cocaine hydrochloride
salt into a base form that has been freed of hydrochloric
acid. e key difference lies in the final stages of the process;
making “freebase” involves an additional extraction step
by means of an organic solvent to eliminate impurities,
thus resulting in a purer form of cocaine than “crack.”
is method is dangerous because of the use of a highly
flammable organic solvent, typically diethyl-ether, that
can ignite if subjected to heat or a flame and may cause
severe burns.
In contrast, “crack” is relatively easy and safe to manufacture
at home from cocaine hydrochloride, which may explain its
Volume 2

4
popularity on many drug markets in Europe, the Ameri-
cas and elsewhere. However, as little or no purification is
involved in its preparation, “crack” usually contains most
of the impurities, diluents and adulterants intrinsic to the
starting material. e purity of the final product in both
“crack” (as found on the European and North American
markets) and “freebase” depends largely on the purity of
the cocaine hydrochloride used as starting material.
Contents of cocaine hydrochloride
products evolve constantly
Cocaine hydrochloride (“powder cocaine”) is the salt most
frequently encountered in cocaine consumer products. It
comes in the form of powder containing varying amounts
of other substances which can be categorized either as impu-
rities (alkaloids, solvents, and cocaine base) or as cutting
agents (diluents and adulterants). While impurities can
constitute up to 10% of the total, cutting agents account
by far for the largest proportion of the non-cocaine mate-
rial found in most cocaine hydrochloride powders. ey
are usually added along the illicit distribution chain to
increase product volume and profits. Unlike precursors and
essential chemicals, cutting agents are typically not subject
to international control, although some may be subject to
controls under national health and/or food regulations.
Impurities and cutting agents come from three different
sources: the plant material used to manufacture cocaine
hydrochloride; the cocaine manufacturing process; and the
process of dilution and adulteration. Combinations and
concentrations change over time as illicit manufacturing
processes evolve in response to changes in global cocaine
market.
(Bi)carbonates and sugars, most
popular diluents in South America
and Europe respectively
Diluents are inert, pharmacologically inactive substances,
many of which are routinely used in the food industry and
can be purchased with relative ease at comparatively low
prices. e available literature suggests that the cocaine
diluents most frequently found in South America are car-
bonates and bicarbonates, whereas those most frequently
used in Europe are sugars. e process of diluting cocaine
hydrochloride is more likely to occur in transit and con-
sumer countries than in producing countries.
Levamisole and phenacetin, most
frequently found adulterants
Most of the adulterants found in cocaine hydrochloride
powders are pharmaceutical drugs, and they tend to be
more expensive and harder to procure than diluents since
they may be subject to more national controls. Local anaes-
thetics appear to be the substances with the longest history
of use as cocaine adulterants, while levamisole (a substance
widely used in veterinary medicine) and phenacetin (an
analgesic) appear as those most frequently found in the
last 10 to 15 years. Many cocaine adulterants pose signifi-
cant health risks as they amplify the toxicologic effects of
cocaine. More rarely, adulterants are other illicit drugs like
amphetamine and methamphetamine, and even more rarely
they include new psychoactive substances.
Adulterants have diversified but
cocaine purity has increased
e range of adulterants found in cocaine hydrochloride
samples has increased since the 1980s, and especially since
the early-2000s, in Europe and the Americas. However,
there are indications that adulterant concentrations in
cocaine powders have decreased recently both in source
countries and in destination markets, while cocaine purity
has increased. ese developments reflect a greater avail-
ability of cocaine in the global cocaine market in the 2010s.
COCAINE INSIGHTS

5
Policy implications
D
espite the fact that cocaine is extracted from
a natural origin, the cocaine-based products
bought by consumers worldwide differ in sig-
nificant ways.
Beyond the chemical nature of the primary psychoactive
substance–which can take on two main forms (base and
salt)–the variability also lies in the additives, impurities
and residues present alongside cocaine; these factors taken
together determine important properties such as the physi-
cal characteristics, possible routes of administration (mainly
insufflation, smoking and injecting), the purity levels and
the potential for harm. In practice, the derivation of the
product is also crucial in order to fully understand its char-
acteristics. Hence cocaine, as a drug, needs to be understood
not just as a substance, but rather as a spectrum of products.
Authorities engaged in drug supply reduction and drug
demand reduction must be wary of reducing cocaine
products found in the illicit consumer markets to a single
substance. e different forms of cocaine reflect, directly
or indirectly, different realities in terms of the supply chain
as well as the potential level of harm posed for health, and
the threat can be best addressed if it is properly under-
stood. It is important to appreciate that cocaine products
undergo a chain of processing steps which often extends
beyond the country of cultivation and even sometimes
involves the consumers themselves. For instance, although
crack [BR], crack [FCP] and coca paste (PBC) contain the
same substance as their main psychoactive ingredient, their
presence in a given country could suggest very different
market dynamics.
In order to ensure that practitioners in drug supply and
drug demand reduction fully appreciate these distinctions
and their implications, and can capitalize on the insights
they bring, there is a need for awareness raising and sen
-
sitization among relevant personnel. Moreover, records
of seizures and other law enforcement interventions need
to differentiate between the different cocaine products to
the extent possible, and data collection mechanisms must
be set up accordingly, so as to enable proper evidence-
based strategies and programmes. In the absence of such
reliable distinctions, there remains a risk that conflations
and inaccurate naming of cocaine products will hinder
the understanding of the cocaine market, and hence the
response to its threat.
Forensic laboratories need to be adequately equipped,
and have the capacity, not only to identify the presence of
cocaine, but to profile the product holistically, including
the chemical form (base or otherwise), purity levels, the
nature of additives, trace alkaloids, solvent residues, isotope
ratios, etc. ese additional parameters provide a signature
on the origin and history of the product, and may thus shed
light on the country of cultivation, sequence of steps and
methods used, and thus potentially on the routes and actors
involved. Hence, understanding the nature of cocaine
products ultimately helps to counter cocaine trafficking and
the cocaine market. For example, some accounts point to
an ongoing proliferation of certain stages of the production
process of cocaine products beyond the countries where this
activity is well-established. In order to confirm and fully
understand this phenomenon, and to counter the associated
threat, the collection, processing and dissemination of data
on forensic profiles and production practices need to be
refined to capture the differentiations between the different
cocaine products outlined in this document.
For instance, systematically distinguishing between the
various products containing cocaine in base form may shed
light on the extent to which, and how, such products are
being trafficked internationally to serve as starting material
for processing in transit and destination countries.
Understanding and differentiating cocaine products and
associated use patterns are equally important for demand
reduction services in view of the different potential for
harm associated with the various routes of administration
and the various adulterants and residues.
ere is a certain regional character to the global variability
of cocaine products. It is no coincidence that the manu-
facturing process consumer products (MCPs) are mainly
found in South America (close to the production areas).
e fact that these products are mainly smoked may have
rendered smoking a more common route of administration
of cocaine than in other markets, and may have set the
scene for the consumption of other smokable products in
this region as well as in neighbouring Central America and
the Caribbean. us, the apparent proliferation of cocaine
processing activity, which seems to affect countries in Africa
and Europe in addition to Latin America, has the potential
to contribute to an expansion of the market of smokable
products in these regions and to developments of use pat-
terns similar to those in South America; this comes in the
context of signs of an increase in the smoking of cocaine
products (specifically crack [FCP]) in Europe.
Ultimately, a better differentiation of cocaine products
in the international discourse on illicit drug markets will
enable a better understanding of the phenomenon, an abil-
ity to interpret and anticipate the developments in the
cocaine market and a more effective and more pre-emptive
response to the problem.
Volume 2

6
C
ocaine consumption is a global phenomenon
present in all world regions, albeit with varying
degrees of intensity. In 2019, 20 million people
were estimated to have used cocaine in the past
year (UNODC, 2021a). Multiple indicators, ranging from
cultivation to seizures and use, point to an ongoing expan-
sion of the global cocaine market. Yet the understanding of
this market is still uneven, with some aspects still subject
to open questions, misconceptions, conflation of concepts
and reliance on anecdotal information.
is document aims to shed light on what the cocaine
consumer products are, including how they are prepared
and what substances they contain, and in particular whether
their main active ingredient is cocaine in the salt (typically
hydrochloride) or the base form. However, this informa-
tion alone is not sufficient and must be combined with
information on how these products are consumed – the
routes of administration used by consumers – as these have
a critical bearing on the effects felt by the users and, cru-
cially, the harms they may cause.
However, data are not available for all the affected regions
and relevant products and those that are available are often
difficult to access. In addition, the nature, quality, reliabil-
ity and timeframe of the information available can vary,
often widely, leading in particular to comparability issues.
As a result, publicly available information on cocaine can
sometimes be confusing or contradictory. And knowledge
gaps remain in many world markets regarding the cocaine
products available to users, in terms of their chemical form,
purity, cutting agents used for dilution and adulteration,
price and routes of administration. Both cocaine supply
and cocaine demand are highly dynamic, marked by fre-
quent shifts and changes. is has been especially visible
in the early 2020s, where a combination of exceptionally
high levels of cocaine production, intense growth in trans-
portation and logistics chains globally (EMCDDA and
Europol, 2019; 2016), a diversification of criminal actors
involved in the supply chain from South America to Europe
(UNODC and Europol, 2021b) and near-ubiquitous access
to internet-based technologies has probably made more
cocaine products available to more potential consumers
in more countries than ever before.
e cocaine market is a considerable source of harms in
terms of both security and health. In 2019, cocaine use
disorders accounted for an estimated 1.15 million healthy
years of life (DALYs) lost due to disability (559,000) or
premature death (594,000) (IHME, 2021).
Cocaine use also contributes to the spread of infectious
diseases through the sharing of smoking equipment as well
as syringes, and as a risk factor for unsafe sexual behaviour
(UNODC, 2017; Ti et al., 2011; Johnson et al., 2016).
Certain cocaine use patterns have been particularly associated
with marginalization and poor socioeconomic conditions
(UNODC, 2016b); they may also contribute to acquisitive
crime and violent behaviour (UNODC, 2019c). In some
countries along the cocaine trafficking routes, large-scale
trafficking of the drug occurs in parallel with high levels of
violence (UNODC, 2016b; UNODC, 2019c).
is report offers an overview of the cocaine consumer
products available at global level. e illicit production
chain is discussed insofar it sheds light on the consumer
products. e report is based on available published evi-
dence and on the knowledge gained through UNODC’s
monitoring activities in South American countries.
e report discusses briefly some chemical and pharma-
cokinetic aspects of cocaine, and then proceeds to define
and discuss three main families of consumer products:
manufacturing process consumer products (MCPs) derived
from cocaine base and coca paste (PBC); freebase consumer
products (FCPs) derived by converting cocaine salt back
to base form; and consumer products based on cocaine
hydrochloride (typically in powder form).
Introduction
COCAINE INSIGHTS
7
The cocaine production
process
W
hile this report focuses mainly on cocaine
products which are consumed by users, the
manufacturing process itself is important
in understanding these consumer products.
e process from coca leaf to cocaine hydrochloride, the
main end-product ready for export in wholesale quantities,
itself involves some intermediate products, namely coca
paste (PBC) and cocaine base.
e manufacturing of cocaine hydrochloride is a dynamic,
adaptive process that varies depending on the context in
which it takes place. Geography, developments in agricul-
tural methods, availability of the raw material and of the
chemicals needed for the manufacturing process, drug traf-
ficking routes and the presence of armed groups are some
of the factors reported to have a major impact on how
and where cocaine is produced in Bolivia, Colombia and
Peru. How these factors combine at different times within
particular regions in the three countries goes a long way to
explain why different methods of cocaine production have
been documented to exist.
at said, although the methods vary, all cocaine production
processes are centred around 4 clearly differentiated prod-
ucts: coca leaf; coca paste (PBC); cocaine base; and cocaine
hydrochloride (SIMCI, 2019b). All of these are commercial
products, that is, they are bought and sold among actors of
the cocaine manufacturing industry and one or several mar-
kets exist for each one of them. In this report, these products
are referred to collectively as “products of the production
process” in order to differentiate them from the “consumer
products” sold to individual cocaine users that are reviewed
in Section 3 of the present report. is section provides a
brief overview of what is known about the 4 products of
the production process.
e taxonomy used in this report relies on three major
criteria: (1) the chemical form (base or salt) of cocaine
present in the product; (2) the sequence of intermediate
products from which the end-product is derived; (3) the
specific methods and additional chemicals used to extract,
convert, purify, adulterate and dilute the substance.
On the basis of criterion (1), the first major class of consumer
products which can be identified are the hydrochloride-based
powders. Aside from these, the consumer products contain-
ing cocaine in base form can be distinguished, on the basis of
criterion (2), into two major classes, namely: the consumer
products which are derived from intermediate stages of the
production process from coca leaf to cocaine hydrochlo-
ride (the MCPs); and those which are derived from cocaine
hydrochloride (the FCPs). Criterion (3) is then further used
to distinguish between different FCPs and different MCPs.
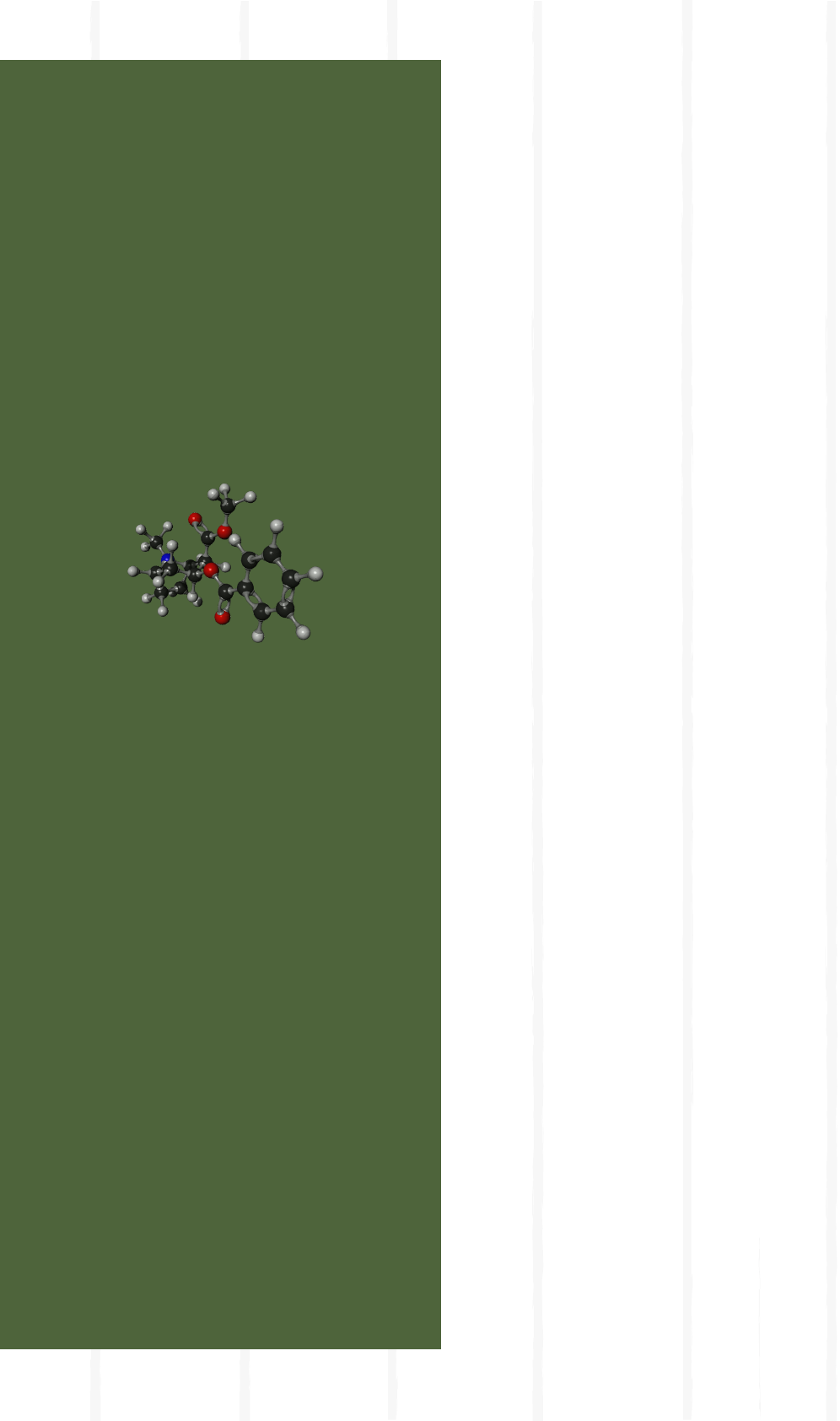
What is cocaine?
Basic information
Cocaine is a natural substance occurring in
the leaves of certain plants native to South
America. Cocaine was placed under interna-
tional control, along with the closely related
substance ecgonine, by the Single Convention
on Narcotic Drugs of 1961 as amended by the
1972 Protocol. While the convention defines the
“coca bush” as any plant of the genus Eryth-
roxylon, which is comprised of more than 250
species, cocaine is in practice extracted from
the leaves of two cultivated species: Erythrox-
ylon coca and Erythroxylon novogranatense
(each of which occurs in two varieties).
Cocaine belongs to the family of substances
called alkaloids. From a strictly chemical point of
view, “pure” cocaine may occur in two forms:
base and salt. The cocaine base molecule
(C
17
H
21
NO
4
, benzoylmethylecgonine) consists
of the “heart” of the drug and accounts for its
psychoactive effects, which include a sense
of physical and mental well-being, exhilaration
and euphoria. Cocaine in base form is available
on consumer markets in many world regions;
it is mostly smoked. Cocaine salts consist of
larger molecules and, theoretically, can come
in several kinds, such as cocaine hydrochloride,
cocaine nitrate, cocaine sulphate, and several
others. However, in practice, cocaine hydro-
chloride is the salt which is most frequently
encountered as an end-product sold to con-
sumers. Cocaine sulphate may occur in the
intermediate products of the cocaine produc-
tion process.
Given that there are no indications of synthetic
cocaine illicitly manufactured (or diverted) on
any consequential scale, it may be assumed
that the cocaine consumer products available on
global markets have been manufactured from
coca leaf. However, it is important to bear in
mind that such products typically do not consist
of a “pure” substance and therefore aspects
such as impurities, physical characteristics and
methods of production and consequent resi-
dues are part of their defining characteristics.
Three-dimensional rendering of the cocaine
base molecule
Volume 2

8
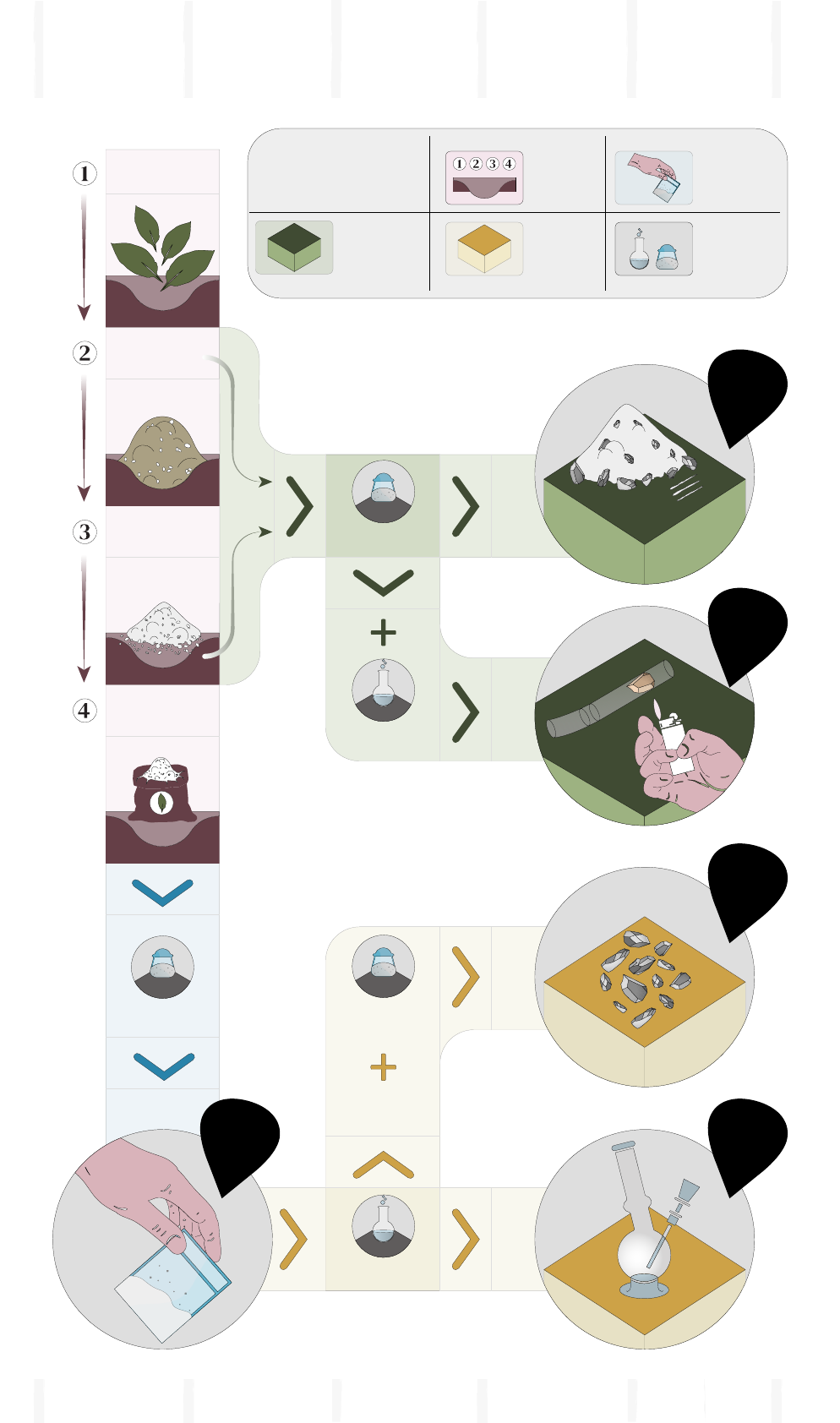
FIG. 1
The cocaine manufacturing process
(
1) Additional steps may be required for some of the products sold to end consumers.
(2) In accordance with the laws in Peru and Bolivia, a legal market also exists for the sale of coca leaf for traditional consumption purposes. In the case of Colombia, produc-
tion of coca leaf is aimed predominantly for the illicit production of cocaine; traditional use is limited.
Source: UNODC SIMCI, Colombia; elaboration based on various studies related to coca cultivation and the manufacture of cocaine
hydrochloride.
PRODUCTION
PROCESSES
CHEMICALS
AND INPUTS
TRADEABLE
PRODUCTS
(available on illicit market)
Cocaine
paste (PBC)
Pasta bruta
de cocaina
Fertilizers
Pesticides
Herbicides
Sulphuric acid
Potassium
permanganate
Organic solvents
Salts, Bases
Fresh coca leaf
Dry coca leaf
Cocaine
hydrochloride
Cocaine
base
Pasta básica
lavada
Cultivation of coca bush
Extraction process
Refining/ oxidation/
“washing”/ purification
conversion/ crystallization
Hydrochloric
acid
Organic
solvents
Salts
Sulphuric acid
Organic solvents
Alkaline
substances
COCAINE INSIGHTS

9
Naming cocaine products for clarity
The information on cocaine products available in the
literature is often ambiguous, unclear and can ulti-
mately be confusing. A serious problem frequently
encountered when attempting to describe cocaine
products is the fact that products are named but
that no definition of what they are is explicitly pro-
vided. The definitions are often left implicit, as if they
were obvious and necessarily meant the same thing
for everyone regardless of national particularities,
cultural specificities and language.
In an effort to begin clarifying what the different
cocaine products reviewed here are and are not,
a specific terminology has been adopted in order
to name as precisely as possible the different
products identified in this report. The terminology
complements the taxonomy of cocaine products,
as described in Figure 2, to convey the nature
of the products. The terminology is reflected in
a typographical convention used throughout the
report in an effort to remove some ambiguities.
In the present report:
•
Quotation marks (e.g. “crack”, , ‘Oxi’) are used to
mark words or passages as they appear in a bib-
liographical source and/or to indicate uncertainty
as to the exact nature of the cocaine product
between the quotation marks.
•
Other terms (not in quotation marks) in a lan-
guage other than English are in italics.
Moreover, the following nomenclature and typo-
graphical conventions have been adopted:
refers to a smokable MCP
reported by Colley and
Casale (2014) to have been
manufactured and sold to
consumers for many years in
Bolivia, Colombia and Peru
Crack
[SA]
refers to a smokable FCP
frequently found in the
United States and Europe,
among other markets
Crack
[FCP]
a street name used to refer
to a range of mostly
smokable MCPs found in
several South American
countries
‘Pasta
base’
a street name used to refer
to a range of mostly
smokable MCPs found in
Argentina and Uruguay
‘Paco’
a street name used to
refer to a range of mostly
smokable MCPs found in
Colombia and Venezuela
‘Basuco’
refers to a smokable MCP
found in Brazil
Merla
‘PBC’
a street name used to
refer to a range of mostly
smokable MCPs found in
several South American
countries
Crack
[BR
]
refers to a smokable MCP
found in Brazil
once erroneously said to be
a new individual cocaine
product, ‘oxi’ is a street name
used to refer to a range of
cocaine products available
on the Brazilian market
‘Oxi’
a product of the cocaine
manufacturing process.
Often also referred to as pasta
básica de cocaína, or
cocaine base paste– hence
the acronym PBC.
Coca
paste
(PBC)
South
America
South
America
Argentina,
Uruguay
South
America
Brazil
Brazil
Brazil
Colombia,
Venezuela
Bolivia,
Colombia,
Peru
USA,
Europe
Volume 2
10
FIG. 2
Schematic representation of the relationship between the different cocaine products
LEGEND
Products of
the cocaine
production
process
Hydrochloride-
based
powders
Freebase
consumer
products
(FCPs)
Additional
operation
Manufacturing
process
consumer
products (MCPs)
OPTIONAL
ADULTERATION
CHEMICAL
PROCESS
CHEMICAL
PROCESS
OPTIONAL
ADULTERATION
cocaine base
coca leaf
coca paste
cocaine
hydrochloride
Hydro-
chloride-
based
powders
OPTIONAL
ADULTERATION
‘Basuco’,
‘Oxi’,
‘Paco’,‘PBC’,
etc.
Crack [FCP]
- Crack
[BR] [SA]
- Merla
Freebase
COCAINE INSIGHTS

11
Products of the cocaine
production process
e coca plant is the only natural source of cocaine. Aside
from some wild-growing species whose leaves contain very
small quantities of cocaine, the natural cocaine alkaloid is
mainly found in the leaves of a range of cultivated variet-
ies, or cultivars, of the plant that are grown mostly on
the eastern slopes and valleys of the Andes and in some
Amazonian lowland regions of South America (Plowman,
1981). However, cocaine is only one of several alkaloids
present in coca leaves (Rivier, 1981).
Coca paste (PBC) is the first alkaloid-rich intermediary
commercial product obtained when manufacturing cocaine
hydrochloride from coca leaf (see Figure 1). Some sources
(UNODC, 2016a; UNODC, 2012; ElSohly et al., 1991)
refer to this product as “coca paste”, but it is also frequently
known as pasta básica de cocaína (PBC), or “cocaine base
paste”. However, the latter term can be misleading as this
product may contain cocaine sulphate, which is a salt of
cocaine (rather than a base). Nevertheless, given the wide-
spread use of the term pasta básica de cocaína, this document
henceforth refers to this product as “coca paste (PBC)”.
It is sometimes also known as base paste (pasta base) (OUD,
2014). In Brazil, this product is known in Portuguese as
pasta base de cocaina (Da Silva Júnior et al., 2012), or simply
as pasta base (Campos Neto, et al., 2012). In Peru, the first
alkaloid-rich product obtained when processing coca leaf
has sometimes been called pasta bruta de cocaína or pasta
cruda de cocaína (“crude cocaine paste”).
Cocaine base is the second commercial intermediary
product between coca leaf and cocaine hydrochloride (see
Section on Chemical forms of cocaine below). It is obtained
by purifying coca paste (PBC), and as a result its cocaine
content is higher than that of coca paste (PBC), being esti-
mated at about 80% in Colombia. Its sale price is superior
to that of coca paste (PBC) (SIMCI, 2019b).
Within coca paste, cocaine is already present predominantly
in base form,
1
alongside other substances. Some of these
substances can be removed by the process of oxidation,
which is achieved by adding a dilute acid and potassium
permanganate to coca paste, yielding the purer product
referred to as cocaine base. However, in Peru, purification
of pasta cruda de cocaína has been reported to be most
frequently performed with an alcohol, especially ethanol,
and the resulting product is known as pasta básica lavada
(“washed base paste”) or pasta base oxidada (“oxidized base
paste”),
2
or simply base lavada (“washed base”) (Casale et
al., 2008a).
Cocaine hydrochloride (HCl) is a salt that is produced
by crystallisation of cocaine base with hydrochloric acid
(see Section on Chemical forms of cocaine below). It is
the end-product of the manufacturing process, and it
is the main ingredient in the products commercialized
globally, in myriad of different wholesale, semi-wholesale
and retail markets.
1 Given the imperfect processes in clandestine operations, and variations in the
production process, coca paste may also contain cocaine in salt form (cocaine
sulphate).
2 UNODC Illicit Crop Monitoring Programme, Colombia (SIMCI).
cocaine base
coca leaf
coca paste
cocaine
hydrochloride
Volume 2

12
Consumer products
Chemical forms of cocaine
products, routes of administration
and basic pharmacokinetics
T
he cocaine alkaloid extracted and isolated from
coca leaf is a chemical base (Benowitz, 1993).
However, it is made available on world con-
sumer markets in two chemical forms: as a base
(with minimal solubility in water) and as hydrochloride salt
(soluble in water) (Wexler, 2014).
3
A range of consumer
products is derived from each of these forms.
Both chemical forms are readily absorbed through all
mucous membranes of the body, including the mouth,
nose, lungs, stomach and intestine (Karch and Drummer,
2015). Nonetheless, the chemical properties of each form
partly determine the routes of administration available
to users. In turn, routes of administration determine to a
considerable extent the effects of cocaine on the body and
the severity of the physical and psychological harms that
can result from use (Karch and Drummer, 2015). By far the
most frequently used routes of administration at global level
appear to be the nasal insufflation (“sniffing”, “snorting”)
of products in which cocaine is in hydrochloride salt from,
and the inhalation of the vapours when products containing
cocaine in base form are smoked.
4
e smoking of cocaine
base products is likely to result in more harms to users than
the snorting of the hydrochloride salt (Hatsukami and
Fischman, 1996; WHO and UNICRI, 1995).
Cocaine base is readily smokable as it starts to vaporize at
a relatively low temperature of around 90°C (UNODC,
2013; Dujourdy et al., 2010; Lizasoain et al., 2002;
INCHEM, 1993). Consumer products containing cocaine
base as the main psychoactive ingredient are smoked
5
in
a variety of ways, including in ad-hoc pipes, in tobacco
and cannabis cigarettes, through vaporization on an alu-
minium foil (“chasing the dragon”), in electronic cigarettes
and using makeshift equipment improvised from common
3 The solubility of cocaine base and cocaine hydrochloride in water are
estimated at 0.17g per 100ml and 200g per 100ml respectively.
4 Based on data from 27 countries worldwide which responded to the
relevant question in the UNODC Annual Report Questionnaire for
2019, the proportion of users who injected the drug was on average
8.7 per cent in the case of cocaine salts and 6.9 per cent in the case of
“crack”[FCP].
5 In the mid-1980s, some users in the United States were reported to
insufflate cocaine freebase nasally (Adams and Kozel, 1985). However,
this was quite rare at the time and it has not been found reported as a
method of cocaine base intake in recent years, although it is likely that
some present-day users insufflate cocaine base without being aware that
it is cocaine base (Dujourdy et al., 2010).
This section focuses on what is known about
the cocaine consumer products available in
the different world regions in terms of chemi-
cal forms, routes of administration, range of
products, purities and cutting agents used.
items such as cups and cans (Bastos and Bertoni, 2014;
CICAD, 2014; UNODC, 2017; Release, 2020). By con-
trast, cocaine hydrochloride melts at 195°C, will decompose
before vaporising and is thermolabile, meaning that it loses
its properties when heated; therefore, it is not adapted for
smoking (Colussi-Mas et al., 2003; Lizasoain et al., 2002;
Benowitz, 1993; Stinus, 1992; Siegel, 1982)
6
. is goes a
long way to explain why the most prevalent route of admin-
istration of cocaine hydrochloride is nasal insufflation.
Consumer products derived from cocaine base may appear
in a diverse range of colours and textures (TNI, 2019;
Henman, 2015), although the most commonly found prod-
ucts include off-white, grey or yellowish chunks of waxy,
translucent solids often reminiscent of gravel or small rocks.
is aspect is at the origin of some of the many different
“street names” given to cocaine base products, including
pedra in Portuguese, piedra and roca in Spanish, rock and
gravel in English, caillou and roche in French, etc. e
term “crack”, probably the most widely known name of a
cocaine base consumer product, originates in the popping
sound often produced when heating cocaine freebase in
order to smoke it. Although originally from the English
language, the term “crack” is now used to describe cocaine
base products in many non-English-speaking drug markets
around the world.
e cocaine hydrochloride salt made available to consumers
worldwide often appears as a white or off-white crystalline
powder but may also presents itself as white shiny flakes or
as a piece of solid material. Products based on the cocaine
hydrochloride form are typically crushed into a fine powder
before they are insufflated.
Being a salt, cocaine hydrochloride is readily soluble in
water, and can therefore be injected in an aqueous solu-
tion. By contrast, cocaine base must be mixed with a weak
acid such as vinegar or lemon juice in order to be dissolved
and become injectable. When injected, both forms can be
used on their own or in combination with other drugs,
frequently heroin (“speedball”). In Europe, for instance,
recent studies of residues in used syringes suggest that when
cocaine is injected in combination with another drug, it is
most frequently with heroin, although instances of cocaine
combined with buprenorphine, methadone or, to a lesser
extent, a cathinone were also found (EMCDDA, 2019a).
In addition, cocaine hydrochloride and cocaine base may
also be used orally, either by eating, rubbing against the
gums or placing under the tongue.
While data on the route of administration of cocaine
products are not systematically available at global level, the
available data, mostly partial and indirect, indicate that
nasal insufflation of cocaine hydrochloride and smoking
6 Casale and Klein (1993) note that the melting points of pharmaceutical
grade cocaine base and cocaine hydrochloride are respectively 98°C and
195°C but that illicitly produced versions are likely to have lower melt-
ing points due to the presence of impurities.
COCAINE INSIGHTS

13
of cocaine base products are the most common routes of
administration, followed by injection and lastly by oral
use.
7
Cocaine hydrochloride tends to be the most widely
used cocaine product in most countries and, as mentioned
previously, it does not lend itself to smoking. Moreover,
the number of users of cocaine hydrochloride in a given
country typically exceeds the users of any other cocaine
product, and among these, only a minority inject (the
same holds for users of other types of cocaine), leaving
nasal insufflation as the primary route of administration
of cocaine salts. Out of 14 countries worldwide
8
which
reported prevalence of use of cocaine salts and at least
one additional (smokable) type of cocaine through the
UNODC Annual Report Questionnaire for 2019, the data
for 13 countries indicated that the number of past-year
users of cocaine salts was more than double the number
of users of any other type.
9
Moreover, based on data from
27 countries worldwide which responded to the relevant
question in the UNODC Annual Report Questionnaire
for 2019, the proportion of users who injected the drug
was on average 8.7 per cent in the case of cocaine salts and
6.9 per cent in the case of crack [FCP].
European data indicate that, among cocaine users enter-
ing treatment in 2018-19 who reported the main route of
administration, 69% used nasal insufflation, 26% smok-
ing (inhalation), 2.3% injection and 1.7% ingestion
(EMCDDA, 2021).
e onset of action and the peak and duration of the
effects depend on the dose administered and on the route
of administration as these determine how much of the
drug will enter the bloodstream and reach the brain, and
how fast (Bono, 2008; Fattinger et al., 2000; Cone, 1995).
However, the individual characteristics of users will also
influence these factors (Fattinger et al., 2000; Cone, 1995).
Compared to smoking and injection, nasal and oral admin-
istration are estimated to result in slower absorption of
cocaine into the bloodstream and slower onset of action
together with a later peak and longer duration of effects.
Oral ingestion appears to have the lowest bioavailability of
all routes, with 60% to 70% of the cocaine estimated to be
destroyed by the body and producing no effects (Karch and
Drummer, 2015; UNODC, 2013; Lizasoain et al., 2002;
Fattinger et al., 2000). e effects of the remaining 40%
to 30% peak within 30 minutes and last up to 2 hours.
When cocaine is insufflated nasally, effects are estimated
7 Cocaine may also be insufflated or rubbed in the rectum (“plugging”),
vagina and penis, often in order to enhance sexual pleasure, but these
routes are even less frequently reported than oral administration. Acciden-
tal administration of large amounts of cocaine in the bowels, rectum or
vagina occasionally occurs in drug couriers transporting the drug intracor-
poreally, which may lead to fatal overdose (Karch and Drummer, 2015).
8 The geographical distribution of these countries was as follows: 6 in
South America, 1 in Central America, 1 in North America, 1 in Asia
and 5 in Europe.
9 In addition, the only 2 countries which reported data specific to cocaine
products other than cocaine salts without reporting data specific to
cocaine salts, provided aggregate data for cocaine in general which indi-
cates that the users of the relevant “smokable” product comprised no
more than a quarter of the cocaine-using population.
to occur within 1 to 5 minutes, peak in approximately 30
minutes and last for about an hour (Karch and Drum-
mer, 2015; OFDT, 2012; Shannon et al., 2007; Lizasoain
et al., 2002; Perez-Reyes et al., 1982). Estimates of the
bioavailability of cocaine administered by the intranasal
route reported in the literature vary widely between 25%
and 80% (Fattinger et al., 2000), with a study reporting
as much as 94% (Cone, 1995). However, since in many
cases a proportion of the cocaine that is insufflated nasally
is swallowed, it will not become bioavailable via nasal
mucosa but via the digestive system, which complicates
measurement of bioavailability via the nasal route (Fat-
tinger et al., 2000; Cone, 1995).
By contrast, when cocaine is smoked or injected the effects
are felt almost immediately and intensely, producing a
euphoric feeling (“rush”) that is much more intense than
with the oral or nasal routes. e onset of action may occur
slightly more rapidly after vapour inhalation (5 to 10 sec-
onds) than after injection (15 to 20 seconds) but the effects
are reported to peak within 3 to 5 minutes in both cases.
When cocaine is smoked the effects appear to be relatively
short-lived, lasting between 5 and 15 minutes, and are
followed by a sharp drop (“crash”) frequently leading to a
craving for another dose; when injected their duration is
longer at 20 to 60 minutes, but a “crash” effect is also often
felt (UNODC, 2013; OFDT, 2012; Shannon et al., 2007;
Lizasoain et al., 2002; Siegel, 1982).
In a study comparing the pharmacokinetics of different
routes of cocaine administration, the average bioavail-
ability of smoked cocaine was estimated at 70% (Cone,
1995). However, cocaine bioavailability when the drug is
administered by smoking is heavily dependent on a series
of factors, which may vary widely between individuals and
even between smoking sessions by the same individual.
ese factors include the temperature of volatilisation of
the cocaine and the amount of drug loss due to decom-
position and to condensation, which in turn depend to a
considerable extent on the type of smoking device used
and on the skills and experience of the individual using it
(Karch, 2008; Cone, 1995; Perez-Reyes et al., 1982; Siegel,
1982). As for the intravenous route, the biovailability of
cocaine (as all drugs) is by definition 100% bioavailable
(bioavailability is defined by how much of a substance
enters the bloodstream (Karch, 2008)).
e available data indicates that nasal insufflation of
cocaine hydrochloride is how a vast majority of users in
Europe, North America and Oceania use the drug, with
base smoking apparently restricted to a small minority.
However, in the Caribbean and Latin America, while the
data seem to broadly indicate that a majority of users also
snort cocaine hydrochloride, there is evidence to suggest
that a much larger proportion of users smoke cocaine base
products than in other regions (CICAD, 2019a). Some
sources even indicate that in some countries such as Bolivia,
Chile, Colombia and Peru, the majority of cocaine users
Volume 2

14
are smokers of base products, specifically MCPs as they
are named in the taxonomy proposed above (see Figure 2)
(SIMCI, 2019b; Comunidad Andina, 2013; CONACE,
2004). It should be stressed that, at global level, the number
of users of products containing cocaine in base form is
likely to be underestimated since many such users belong
to sectors of the population that, for a variety of reasons, are
underrepresented in surveys (Janssen et al., 2020). Evidence
for the rest of the world is missing or patchy, making it
challenging to provide a reasonably robust comprehensive
description of the situation.
In any case, it is important to note that our image of
the global distribution of cocaine products and routes of
administration is likely to change in future as more of the
drug becomes available globally. A likely consequence of
the current cocaine wave is that some products and related
routes of administration may emerge or expand in markets
where they were previously absent or restricted to limited
numbers of users and a narrower range of products. In
this context, it will continue to be especially important to
improve the research and monitoring of markets for cocaine
base consumer products, especially since they are likely to
be underestimated even as they may generate more harms
than cocaine hydrochloride markets.
Cocaine base: a diverse range of
consumer products
ere is considerable ambiguity and confusion surrounding
the consumer products where cocaine in base form is the
main ingredient. Some issues arise because reliable, rou-
tine information on the composition of the products, and
possible changes affecting them, is missing or is difficult
to find due to a lack of routine forensic analysis in many
countries or inadequate reporting and sharing of the results
of such analysis. Much confusion is due to the fact that
different terms are used in different markets to name what
is essentially the same thing, such as ‘basuco’ in Colombia
and ‘paco’ in Argentina. And, vice-versa, that the same name
may be applied to what in fact are different products. For
instance, the same word, “crack”, is used in order to describe
different products—an FCP found in many markets and
MCPs found specifically in South America (see Figure
2, and relevant sections below). Also, it is not infrequent
for media reports and law enforcement press releases to
name products without any reference being made to their
chemical composition, adding to the general confusion as
to what name corresponds to what product. e picture is
further blurred with media claims that a “new” smokable
cocaine product has emerged when in fact it has not, as in
the case of ‘oxi’ in Brazil.
e following two sections—on MCPs and FCPs—attempt
to disentangle these issues with a view to clarifying the
nature of the cocaine base consumer products currently
available in different international drug markets. is is
done primarily by identifying what cocaine ingredient they
contain and how they were manufactured. Additional infor-
mation on dilution, adulteration and methods of use is also
provided where possible.
e categorisation of cocaine base consumer products (see
Figure 2) proposed here rests on the analysis of recent foren-
sic and other data and information from cocaine producing
countries and major international consumer markets com-
bined with a review of the international literature. Although
an effort has been made to use recent data and information
from as comprehensive a set of disciplinary, language and
geographical sources as possible, some gaps persist. is
is probably inevitable given the complex, dynamic and
expanding nature of the present-day illicit global cocaine
market, which makes current and comprehensive reporting
on consumer products containing cocaine in base form a
challenging endeavour.
e family of consumer products derived from cocaine in
base form may be divided into two categories depending
on the starting material from which they are prepared—the
Manufacturing process consumer products (MCPs) and the
Freebase consumer products (FCPs). What makes the FCPs
distinguishable from their chemical “cousins” the MCPs,
is that they are prepared from cocaine hydrochloride, and
not from one of the intermediary products such as coca
paste (PBC) or cocaine base.
COCAINE INSIGHTS

15
The manufacturing process
consumer products (MCPs)
MCPs are made from coca paste (PBC) and cocaine base,
the two major intermediary products occurring during
the process of manufacturing cocaine hydrochloride from
coca leaf (see Figure 2). In these two products, cocaine is
predominantly in base form and thus amenable for smok-
ing; indeed, the derived MCPs are primarily destined to
be smoked and they are also known as “smokable cocaine
substances” in English and “cocaínas fumables” or “cocaínas
de combustión” in Spanish (CICAD, 2019a, 2016a, 2016b,
2014; Sedronar, 2015; Henman, 2015; TNI, 2019, Suárez
et al., 2014; Castaño, 2000). Although smoking is by far
the most prevalent route of administration used for these
products, some South American users nevertheless inject
them (Bastos and Bertoni, 2014; Suárez et al., 2014).
MCPs first emerged in Andean cocaine-producing coun-
tries some 50 years ago then spread to other regions of
the Americas (TNI, 2019; UNODC, 2013; OGD, 1996;
WHO and UNICRI, 1995). e information available
suggests that the first MCP, then known as “coca paste”,
appeared initially in Peru in the early 1970s
10
, then spread
10 The early 1970s may be reported in the literature as the start of the
emergence of MCPs because the first recorded clinical description of
a patient presenting for issues related to “pasta base” consumption
occurred in a Lima hospital in 1972 (Castaño, 2000), and the Peruvian
police recorded its first seizure of “PBC” in the same year (UNODC,
2013). However, Henman (2015) suggests that the smoking of MCPs in
to Bolivia, Colombia and Ecuador, and subsequently to
Chile and Argentina and probably Venezuela (TNI, 2019;
CICAD, 2003; Castaño, 2000; OGD, 1996; Jeri et al.,
1978). Some users in Caribbean island countries may
also have experimented with MCPs in the 1980s (OGD,
1996; ElSohly et al., 1991), but it is now reported that
crack[FCP] is the cocaine base consumer product most
widely consumed in the region (TNI, 2019; OFDT, 2012;
Klein, 2004; Ragoucy-Sengler et al., 2003; Jekel et al.
1994), although more detailed forensic evidence would
be needed. During the 1990s and 2000s, MCPs further
spread to Brazil, Paraguay and Uruguay and other South
American countries, and probably to some Central Ameri-
can countries. However, it is reported that “crack” may be
the most frequently used cocaine base product in Central
America at present, but as is the case with the Caribbean,
more evidence is required (CICAD, 2019a; 2016; 2014;
2003; Sedronar, 2019; Maldaner et al., 2016; Bastos and
Bertoni, 2014; OUD, 2014; Santis et al., 2007; OGD,
1998; 1996; ElSohly et al., 1991; Cortés, n.d.).
It is possible that this spread was due, at least partly, to the
relocation of some cocaine manufacturing activities out of
the 3 principal Andean producer countries, which made the
Peru could have started in the 1950s or earlier.
cocaine base
coca paste
OPTIONAL
ADULTERATION
CHEMICAL
PROCESS
‘Basuco’,
‘Oxi’,
‘Paco’,‘PBC’,
etc.
- Crack
[BR] [SA]
- Merla
Volume 2

16
products of the manufacturing process from which MCPs
could be derived, namely coca paste (PBC) and cocaine
base, available in locations where they previously did not
exist (INCB, 2010; OGD, 1996). Although there is little
evidence of this at present, there is a possibility of future
further spread of MCPs to other regions where some stages
of the cocaine hydrochloride production process occur and
the raw materials are available, such as Central America,
Mexico, Europe (EMCDDA and Europol, 2019; TNI,
2019) and Africa (Sidiguitiebe, 2016; Leggett, 2002).
What follows is a description of some of the main MCPs
found on South American markets based on the evidence
available from the literature. Not enough evidence has been
found to even attempt describing products available in other
Latin American regions, such as the Caribbean, Central
America and Mexico. In these latter regions, more research
and, in particular, forensic analysis, is clearly needed.
Although comparatively more data exists on South Ameri-
can MCPs, especially those available in Brazil, it should
be stressed that finding reliable, comparable and stable
evidence on the exact nature of the different MCPs reported
to be sold on South American markets is a difficult task.
One factor contributing to this is the use of different
names in different countries to refer to similar products.
A related problem, that is also an indicator of the paucity
of accurate information on the subject, is the widespread
use in the literature of catch-all categories such “cocaine
base paste”, “PBC”, “smokable cocaines” and other col-
lective descriptors for several MCPs available in different
South American countries, which in reality may or may
not all be the same product.
is lack of precision and clarity is due in large part to a
relative dearth of forensic evidence on MCPs, which in
turn may be due to an absence of analyses or to poor or
non-existent reporting of the results of existing forensic
studies, or to both issues.
Although important knowledge gaps remain, the analysis
of the literature indicates that the main products of the
production process from which the various MCPs are pre-
pared are coca paste (PBC) and cocaine base (see Figure 2).
Crack [BR] [SA]
In many countries, cocaine products may be found under
the street name of “crack”. As mentioned previously, the
term “crack” originates in the popping sound often pro-
duced when heating cocaine freebase in order to smoke it.
e main characteristics which are usually associated with
products referred to as “crack” appear to be the fact that
these products are smokable (hence the cocaine does not
occur in salt form) and have a hard, non-friable consistency
– often described as “rocks” (UNODC, 2016a; Zacca et
al., 2014; CICAD, 2016b).
It appears however that there are important differences
across countries, and likely also within some countries,
between the products known as “crack”, notably in the way
they are derived – which is an important criterion used for
the taxonomy adopted in this paper. In particular, it appears
© Erberto Zani / Alamy Stock Photo
COCAINE INSIGHTS

17
that some – though not all - of the products marketed as
“crack” in some countries in South America differ from
“crack” as it is encountered in the main consumer markets
of North America and Europe, in that they are derived from
the base forms of cocaine (prior to conversion into hydro-
chloride), and hence are by definition MCPs according to
the taxonomy of this paper. In contrast, the term “crack”
as used in North America and in Europe generally refers to
a product obtained from cocaine hydrochloride; in other
words, a freebase consumer product (FCP).
Smokable products known as “crack” are commonly found
on the consumer drug market of Brazil, where they have
been available for several decades and given rise to much
media attention and public concern (Ribeiro de Araújo et
al., 2019; TNI, 2019; Bastos and Bertoni, 2014; Fuku-
shima et al., 2014; Vieira Duarte et al., 2009; Mingardi
and Goulart, 2002; WHO and UNICRI, 1995). “Crack”
has been described in a fairly recent large epidemiological
study as the most consumed smokable cocaine product
in Brazil, ahead of “similar” products such as ‘base paste’,
merla and ‘oxi’ (Bastos and Bertoni, 2014). More recently,
Brazil has been described as the largest consumer market
for “crack” in the world (Ribeiro de Araújo et al., 2019). A
smokable cocaine consumer product with the street name
“crack” is also reported to have been available in Uruguay
since the early-2000s (JND, 2013)
11
and in Paraguay since
the mid-2010s (CICAD, 2016a). Both are countries of the
Southern Cone that share borders with Brazil.
While no precise description of the “crack” available in
Paraguay and Uruguay has been found, the “crack” found
in Brazil has been described briefly in Brazilian forensic
studies as cocaine that has undergone a melting process fol-
lowed by cooling and solidification. Hydrochloric acid and
sodium carbonate (an alkaline substance) are mentioned
in connection with this process (Zacca et al., 2014). e
outcome is described as a solid that will be dried, packaged
and sold to consumers in the form of small rocks (pedra)
that can be smoked pure in pipes or crushed in tobacco or
marijuana pipes or cigarettes (Zacca et al., 2014).
is source suggests that, in principle, the starting point for
“crack” in Brazil could be any of coca paste (PBC), cocaine
base or cocaine hydrochloride. However, forensic profiling
by the Brazilian police forces indicates that, every year over
2012-2020, among samples taken from seizures of cocaine
in base form and tested in the context of a dedicated project
(Forensic Chemistry Service, PeQui project), the major-
ity were consistently not oxidized, with this proportion
reaching 97 per cent in 2020. In sharp contrast, among
samples from seizures of cocaine in hydrochloride form
in 2020, 97 per cent were classified as “highly oxidized”
(BFP, 2021a). Moreover, it was assessed that, as of 2021,
among the samples of cocaine in base form, at least 80
per cent exhibited characteristics of “crack” (as opposed to
11 In Uruguay in the 2000s, crack was reported to be known to some users
as “cooked cocaine” (cocaína cocida) (JND, 2013).
other products containing cocaine in base form, such as
merla) (BFP, 2021b). ese data suggest that most “crack”
in Brazil has not undergone the oxidation step, and hence
neither the transition to cocaine hydrochloride, and is thus
derived directly from coca paste.
Other sources (CICAD, 2016b; WHO and UNICRI,
1995) confirm that the term “crack” in Brazil is used, at
least sometimes, to refer to a product which is not derived
from cocaine hydrochloride – although some of these also
suggest that this exists alongside “crack” which is derived
cocaine from hydrochloride (crack [FCP]). In this connec-
tion, it is particularly interesting to note that a joint report
of the World Health Organisation (WHO) and the United
Nations Interregional Crime and Justice Research Institute
(UNICRI) states that two types of “crack” were available in
the city of São Paulo, Brazil, in the 1990s: “pedra”, made
from coca paste (PBC) and thus an MCP; and “casca”,
made from cocaine hydrochloride, which is named “crack
[FCP]” in the present report (WHO and UNICRI, 1995).
us, it appears that a significant portion – if not all – of
“crack” marketed in Brazil corresponds to a product which
is obtained from intermediate products of the cocaine pro-
duction process rather than cocaine hydrochloride; that
is, an MCP rather than an FCP. Henceforth the present
document refers to this product as crack [BR]; however
crack [FCP] may also exist in Brazil.
It is also important to note that large amounts of “melted
cocaine” have been reported to be frequently seized at Bra-
zilian borders in the form of 1-kilo bricks (Zacca et al.,
2014). is could indicate that some of the products sold
as “crack” to consumers in Brazil have been manufactured
abroad, for instance in Bolivia, Colombia and/or Peru
(Colley and Casale, 2014).
Indeed, a product described by American chemists as “South
American crack” (henceforth denoted as “crack [SA]”) is
reported to be manufactured in Bolivia, Colombia and
Peru. DEA chemists carried out a comparative analysis of
samples of what they called “South American crack” seized
in Bolivia, Colombia and Peru, and of “domestic crack”
(henceforth denoted as “crack [FCP]”) seized in the United
States. According to Colley and Casale (2014), crack [SA]
has been made for many years in the three cocaine produc-
ing countries “for local distribution and consumption”.
e two forms of crack examined in this study, [SA] and
[FCP], were reported to be “easily differentiated” due to
their distinct solvent profiles. e method reported by the
DEA to be typically used in the three Andean countries in
order to make crack [SA] is by “melting a crude cocaine
base obtained directly from coca leaves through traditional
illicit processing methods, skimming off the water and most
water-soluble impurities, and allowing the cocaine base to
cool and solidify” (Colley and Casale, 2014, p. 1). Similar
methods were also briefly described elsewhere (TNI, 2019;
Bastos and Bertoni, 2014; UNODC, 2013, Casale et al.
Volume 2

18
2008a; Malpica, n.d.). According to SIMCI, the method
described by the DEA is known as “fritado” in Colombia,
where it is used in order to rid coca paste (PBC) of humidity
before it is sold on to cocaine base manufacturers.
12
As a
result, the crack [SA] described by Colley and Casale may be
what is known in Colombia as a specific form of coca paste
(PBC), i.e. a product of the cocaine production process. e
same product may therefore be sold for different purposes
to either cocaine consumers or cocaine production actors.
It should be noted that Colley and Casale (2014) do not
mention the use of acids or sodium carbonate in the method
they describe, which may differentiate it from the method
described by Zacca et al. (2014), although both methods
involve some heating, cooling and solidification. Mean-
while, Zacca et al. (2014) do not report a solvent profile
that could be compared to that reported by Colley and
Casale (2014).
As a result, as far as manufacturing methods are concerned,
the evidence does not allow to confidently establish that the
two methods described respectively by the Brazilian and the
American forensic chemists are different, although they cer-
tainly share similarities. Yet, although the crack [BR] found
in Brazil and the crack [SA] found in Bolivia, Colombia
and Peru, may or may not be manufactured using the same
method, it is clearly established that both are manufactured
from one of the intermediary products of the cocaine manu-
facturing process, and therefore that both are MCPs.
Given that several methods of making “crack” in South
America could exist, two possibilities emerge that are not
mutually exclusive:
12 UNODC Illicit Crop Monitoring Programme, Colombia (SIMCI).
•
Firstly, it is possible that some of the substance described
as crack [SA] by Colley and Casale (2014) and as dried
coca paste (PBC) by SIMCI is smuggled from Bolivia,
Colombia and Peru to other South American countries
including Brazil where it is seized in 1-kilo bricks and
retailed in the form of small rocks;
•
Secondly, it is also possible that cocaine base is traf-
ficked pressed into 1-kilo bricks from one or several of
the three producing countries and then transformed
into crack [BR] using the method/s described above
in destination countries including Brazil.
For instance, trafficking of “base paste” has been reported to be
fairly intense in the border areas between Brazil’s Mato Grosso
State and Bolivia (Campos Neto et al., 2012). And, as men-
tioned earlier, coca paste (PBC) is also reported to be exported
from Peru to Ecuador, Bolivia, Brazil, Chile, Argentina and
Uruguay, that is, countries where relatively large markets for
MCPs exist (UNODC, 2013). Argentinian authorities report
that “cocaine base paste” is often pressed into bricks before
transportation (Sedronar, 2015). Some cocaine base is also
reported to be seized in Brazil from international traffick-
ers, particularly in the north-west of the country bordering
Bolivia, Colombia and Peru (Da Silva Júnior et al., 2014).
Although “crack” is not reported as a name given to any
cocaine consumer product commonly available in either
Bolivia, Colombia or Peru, both crack [SA] and crack [BR]
could nevertheless be sold to users outside Brazil under
different names, such as ‘basuco’ in Colombia or ‘paco’
in Argentina and Uruguay, for instance. Unless specific
forensic analysis allowing to determine how the MCPs avail-
able in South American consumer markets are prepared,
for instance by establishing their solvent profiles, this will
remain a knowledge gap.
COCAINE INSIGHTS

19
It may also be speculated that some of the consumer prod-
ucts that are sold as “crack” (CICAD, 2014) in Central
American and Caribbean countries could in fact be MCPs
prepared from coca paste (PBC) or cocaine base, as in
Bolivia, Brazil, Colombia or Peru, and not FCPs prepared
from cocaine hydrochloride as in Europe and the United
States (See Figure 2). As in the case of South America,
this will remain a knowledge gap until forensic analysis is
performed on the “crack” available on the retail markets of
Caribbean and Central American countries.
Finally, it is of course probable that crack [FCP] (see Figure
2) is manufactured from cocaine hydrochloride in South
American countries including Argentina (TNI, 2006),
Brazil (Fukushima et al. 2014; WHO and UNICRI, 1995),
Colombia (TNI, 2019; Molina, 2014), Paraguay (CICAD,
2016a), Peru (Henman, 2015) and Uruguay (JND, 2013),
where it would be available to consumers in addition to
MCPs. However, reports of crack [FCP] being available
to consumers in South America are infrequent, and none
of those mentioned earlier in this paragraph are based on
forensic evidence.
In summary, the available evidence indicates that a cer-
tain MCP - crack [BR] - consisting of a solid, non-friable
form of cocaine base, and derived from coca paste (PBC)
(a product of the cocaine manufacturing process), exists
in Brazil. e crack [BR] found in Brazil may or may not
be manufactured in the same way as the crack [SA] made
in Bolivia, Colombia and Peru. But in any case, the crack
[BR] made in Brazil and the crack [SA] made in Bolivia,
Colombia and Peru may be neatly differentiated from
the crack [FCP] available in other markets such as North
America (and Europe) because the latter has a different
solvent profile, is prepared from cocaine hydrochloride and
hence is a freebase consumer product (FCP) according to
the taxonomy proposed here.
Merla
Merla is a cocaine smokable product that has been avail-
able on the Brazilian consumer market for several decades
(TNI, 2019; Vieira Duarte et al., 2009), especially in the
centre and north of the country (Zacca et al., 2014; Neves,
2013; Blickman, 2006). Medeiros et al. (2009) indicate
that merla, also known as “mela” and “mescla” (mixture
in Portuguese), used to be the name given to the residual
sediment left following the processing of coca leaf into
coca paste (PBC) and that contained a small amount of
cocaine, but that subsequently the name merla came to
be applied to a consumer product that is different from
residual sediment. A similar transfer of the name initially
transferred from a cocaine manufacturing by-product to
a range of consumer products appears to have taken place
in the case of ‘basuco’ in Colombia.
Prevalence of merla use appears to have declined in Brazil
in recent years (Zacca, et al. 2014), and in the last national
survey available it is reported to be lower than use of other
smokable cocaine products such as crack [BR], “pasta base”
and ‘oxi’ (Bastos and Bertoni, 2014) (See Section on ‘oxi’
below). Use of merla is reported to be more prevalent outside
of Brazilian state capitals than in these larger urban settings
(Bastos and Bertoni, 2014). Merla has not been reported
to be a name used to describe cocaine products available
to consumers outside Brazil in the literature reviewed here.
Several descriptions of merla can be found in the litera-
ture, and all concur that the product most often is sold
to consumers in the form of a wet, whitish or yellowish
paste, which is smoked, frequently mixed in tobacco or
marijuana cigarettes (De Souza, 2014; Zacca et al., 2014;
Neves, 2013; Medeiros et al., 2009; Blickman, 2006; TNI,
2006). Forensic analysis of merla indicates that it contains
cocaine in base form, large amounts of water (up to 70%)
and of sodium salts including sulphate, carbonate and bicar-
bonate, and residue of the manufacturing process of coca
paste (PBC) or of cocaine base (Zacca et al., 2014; Neves,
2013; Medeiros et al. 2009).
According to a summary description reported by Brazilian
forensic scientists, merla may be obtained from both coca
paste (PBC) and cocaine base treated with a solvent, for
instance a paint thinner, sulphuric or hydrochloric acid and
sodium carbonate. Heating is not reported to be required
in the preparation of merla (De Souza, 2014; Zacca et al.,
2014; Neves, 2013). Forensic profiling of 30 samples in
the late 2000s indicated that cocaine concentrations in
merla can vary widely and that it is likely that the product
is manufactured in Brazil (Medeiros et al. 2009), prob-
ably from imported coca paste (PBC) and cocaine base as
no reports of international trafficking of merla have been
found in the literature.
In summary, the available evidence indicates that the con-
sumer product called merla is a manufacturing process
consumer product (MCP), and namely a wet paste form
of cocaine base made in Brazil from imported coca paste
(PBC) and/or cocaine base. Merla appears to be available
in Brazil only, where it is sold to be smoked on its own or
mixed with tobacco or cannabis herb. However, it is possible
that similar pasty smokable cocaine products are sold under
different names in other South American countries, for
example some of the products sold as ‘basuco’ in Colombia
(UNODC and OAS, 2014). e Brazilian Federal Police
(Forensic Chemistry Service, PeQui project) commented
that, as of 2021, samples of merla were rarely encountered
and merla had been supplanted by crack [BR] in the illicit
market in Brazil (BFP, 2021b).
‘Oxi’
‘Oxi’, also known as “oxidado”, was first reported and
described as “possibly one of the most potent and danger-
ous drugs known” and “a variant of crack” smoked by users
in the State of Acre, in the Amazonian north-west of Brazil,
Volume 2

20
by a harm-reduction organisation and a news media in
May 2005 (Viana, 2005). Although the news caused some
alarm in Brazil and neighbouring countries at the time, it
was subsequently forgotten (Da Silva Júnior et al., 2012).
However, in late 2010 and during 2011, alarming news
concerning ‘oxi’ reappeared in the Brazilian media and in
some international scientific publications (Bastos et al.,
2011), where it was again presented as a “new” and “highly
potent” smokable cocaine product. ‘Oxi’ was described as
a product related to crack [BR] but different from it since
‘oxi’ preparation was said to involve calcium oxide and a
fuel such as kerosene or petrol. Moreover, ‘oxi’ was said
to be cheaper than crack [BR] because it was made from
residue and by-products of crack [BR] preparation (see
also CICAD, 2014).
However, a Brazilian forensic study has shown that these
reports were not based on facts, and specifically that ‘oxi’
was not a new drug but simply a name given to a range of
existing cocaine products arbitrarily categorized as ‘oxi’ (Da
Silva Júnior et al., 2012). Twenty samples seized at retail
level and officially classified as ‘oxi’ by the Civil Police of the
State of Acre, and 23 samples seized in Acre from interstate
or international traffickers by the Brazilian Federal Police
were chemically profiled. Six of the samples submitted by
the Acre police (20%) were found to be cocaine hydro-
chloride, which is a non-smokable cocaine product. ese
samples were not further analysed as they could not possibly
be ‘oxi’, a smokable, allegedly new product. Analysis of the
remaining 14 samples provided by the Acre Police showed
that 4 were crack [BR], 7 were coca paste (PBC) and 3
were cocaine base. Half of these 14 samples contained the
adulterant phenacetin in varying amounts, and no other
adulterant was found. irteen of the samples submitted by
the Federal Police proved to be coca paste (PBC) and the
remaining ten were cocaine base, with 5 of the 23 samples
containing phenacetin. No diluents were found in either
set of samples and the purity of a majority of the samples
was quite high, ranging between 40% and 80%, with a few
samples above 90% (Da Silva Júnior et al., 2012).
e case of ‘oxi’ appears to be similar to the media hype
surrounding the amphetamine tablets sold in the Middle-
East as “captagon” that occurred in much of the world
following the terrorist attacks of November 2015 in the
Paris region (Laniel, 2017). In both cases, lack of reliable
information about the exact composition of a drug prod-
uct that is assumed to be new or specific due to its “street
name”, led to misleading speculations and unnecessary
public concern. e case of ‘oxi’ illustrates again the cen-
trality of reliable forensic information to the understanding
of drug markets.
In summary, ‘oxi’ is not a cocaine product. It is a “street
name” used in Brazil by some official, media and civil
society organisations to describe a range of other cocaine
products including cocaine hydrochloride and MCPs.
‘Basuco’
Originally, ‘basuco’ (sometimes spelt bazuco) used to be the
name given to the residue of the processing of large quanti-
ties of cocaine. e name is often said to be an abbreviation
of the phrase basura sucia de cocaína (dirty cocaine trash)
(e.g. TNI, 2019), but since the suffix uco is often used in
Spanish to form derogatory words from nouns and adjec-
tives ‘basuco’ may simply be understood as a pejorative form
of the noun, base (de cocaína) (Sabogal and Urrego, 2012).
At present, ‘basuco’ is a name given to smokable cocaine
consumer products available in Colombia and in Venezu-
ela (Sabogal and Urrego, 2012; Dávila et al., 2001)
13
, but
Colombia is the only country for which enough infor-
mation has been found. Use of ‘basuco’ is associated in
Colombia with urban poverty and other social problems
such as homelessness, and the drug is generally perceived by
both its users and the public as a “dirty” drug made of toxic
by-products of cocaine manufacturing (Molina, 2014).
Colombian consumers are reported to smoke ‘basuco’ most
often mixed in tobacco or cannabis cigarettes although it
can also be smoked in pipes (Sabogal and Urrego, 2012).
‘Basuco’ has been described alternatively as a dry solid that
may appear as a rough powder or as a small rock (TNI,
2019; 2006; Sabogal and Urrego, 2012), or as a damp
substance (UNODC and OAS, 2014), both of which may
be of different colours (white, off-white, yellowish, greyish,
brownish).
It is also reported that the chemical composition of ‘basuco’
is variable. us, a forensic analysis of 109 representative
samples of ‘basuco’ seized in Colombia in 2010 showed
that caffeine and, to a lesser extent, phenacetin were the
most commonly found adulterants (Sabogal and Urrego,
2012). e cocaine content of the samples was reported
to vary widely between 4% and 70%, although a majority
of samples was found to contain cocaine in concentrations
of between 20% and 50% (Sabogal and Urrego, 2012).
A more recent study of a smaller number of samples of
‘basuco’ (n=16) and cocaine hydrochloride (n=12) con-
sumer products collected by a harm-reduction organisation
in Bogotá, Colombia, in July 2014, has found a similar
combination of adulterants in ‘basuco’ and confirmed its
relatively high purity (38.8% on average) (Molina, 2014).
Molina (2014) also indicates that residues of the fuels (e.g.
kerosene), acids (e.g. sulphuric acid) and potassium per-
manganate used to manufacture the starting materials of
‘basuco’ (see Section on Products of the manufacturing
process above) may also be found in ‘basuco’. It is also worth
mentioning that the study found that ‘basuco’ samples
contained more cocaine and smaller amounts of a narrower
range of adulterants than cocaine hydrochloride samples
(Molina, 2014). Similarly, an analysis of a small number
of “coca paste” samples seized in Bogotá in the early-1990s
13 ‘Basuco’ is also reported to have been found as an adulterant in tablets
sold as ecstasy in Colombia (Comunidad Andina, 2013).
COCAINE INSIGHTS

21
found significant amounts of fuel and potassium perman-
ganate residues and high cocaine concentrations in the
samples (ElSohly et al., 1991).
It is impossible to ascertain that ‘basuco’ is a specific product
since there seems to be no consensual definition of the term
in the literature reviewed here. Most sources do seem to
agree, however, that ‘basuco’ is either prepared from coca
paste (PBC) (see Figure 2) or is itself coca paste (PBC) in
dry or damp form, with adulterants reported to be added
in both cases (TNI, 2019; CICAD, 2016a; 2014; Fischer et
al., 2016; UNODC and OAS, 2014; Comunidad Andina,
2013; UNODC, 2013; Sabogal and Urrego, 2012; TNI,
2006; Castaño, 2000; Malpica, n.d., etc.). Cocaine base
(see Figure 2) is very rarely mentioned as potential start-
ing material for ‘basuco’ in Colombia, although the cases
of crack [BR] and merla reviewed above make it clear that
this could be an option (De Souza, 2014; Zacca et al.,
2014; Neves, 2013), as does the presence of potassium
permanganate residue.
e reliability of this information is difficult to assess since,
unlike crack [BR] and merla in Brazil and the South Ameri-
can crack [SA] described by the DEA (Colley and Casale,
2014), no description of ‘basuco’ preparation methods has
been found anywhere in the literature, and there are appar-
ently no reports on the solvent or alkaloid profiles of this
product. is may be because no specific forensic studies
have been performed (or their results reported).
us, in the early 2010s, Sabogal and Urrego (2012)
explained that although Colombian authorities differen-
tiated between coca paste (PBC), cocaine base, ‘basuco’
and cocaine hydrochloride when they reported seizures
14
,
14 The Colombian Drug Monitoring Centre recently reported seizure sta-
tistics where ‘basuco’ is differentiated from cocaine hydrochloride and a
the criteria that they used in order to distinguish between
the products were not based on chemistry but on visual
and contextual aspects. ese included whether a seized
substance appears as a finished product (for instance, a
consumer product of about a gram wrapped in a piece of
newspaper; or a larger amount of a dry substance com
-
pacted as a brick); and the locale and circumstances of
the seizure. Importantly, the authors explained that the
Colombian authorities reported that due to “laboratory
limitations” it was not possible to differentiate between
coca paste (PBC), cocaine base and ‘basuco’ in other ways
(Sabogal and Urrego, 2012).
It should also be observed that none of the various reports
about smokable cocaine consumer products, including
‘basuco’, in South America published after 2014 that have
been reviewed here contains any reference to the forensic
study by Colley and Casale (2014) reporting that “South
American crack” [SA] has been made in Bolivia, Colombia
and Peru “for many years”. is is surprising since there is a
strong probability that some of the products sold as ‘basuco’
in Colombia (and Venezuela) could in fact be crack [SA]
(“South American crack”), especially in view of the fact
that no other product reported seized or consumed in the
country could fit the description of crack [SA].
In summary, ‘basuco’ is in all likelihood a “street name”
used to describe a range of different smokable cocaine
substances available on the Colombian (and Venezuelan)
consumer markets. Neither a clear definition of ‘basuco’
nor any description of how such a product could be made
was found in the literature. In this sense, ‘basuco’ would be
similar to ‘oxi’ in Brazil (see above). e available evidence
further suggests that the products collectively referred to
as ‘basuco’ are MCPs (See Figure 2) that contain a fairly
large amount of cocaine and that are adulterated mostly
with caffeine and phenacetin. Although there is not enough
forensic evidence to draw definite conclusions, it is likely
that the term ‘basuco’ as used in Colombia covers the fol-
lowing consumer products:
•
Coca paste (PBC), dried or damp, sometimes adulter-
ated with caffeine and phenacetin;
• Cocaine base, dried or damp, sometimes adulterated
with caffeine and phenacetin;
• Crack [BR] and crack [SA], as described in Brazil and
in Bolivia, Colombia and Peru, sometimes adulterated
with caffeine and phenacetin.
Additionally, merla, as described in Brazil, may also be one
of the products sold in Colombia under the name ‘basuco’,
while crack [FCP] made from cocaine hydrochloride may
also be sold and/or prepared by users in Colombia.
category called “cocaine paste/base” but did not explain how the distinc-
tion was made (ODC, 2017). SIMCI (2019b) uses similar categories.
© Jan Sochor / Stockimo / Alamy Stock Photo
Volume 2

22
‘Paco’, ‘pasta base’, ‘PBC’, etc.
‘Paco’ is a street name frequently used to describe a range
of manufacturing process consumer products (MCPs) that
is mostly smoked in home-made pipes since the early-
2000s by consumers in Argentina and Uruguay (JND,
2019; TNI, 2019; 2006; CICAD, 2016a; Moraes et al.,
2015; Sedronar, 2015; 2007; Arias et al., 2014; Súarez et
al., 2014; Capece, 2008; Míguez, 2008). e term is likely
to be an abbreviation of pasta de coca (“coca paste”) or of
pasta de cocaína (“cocaine paste”).
Many sources addressing Argentinian and Uruguayan drug
issues equate ‘paco’ and terms such as pasta básica de cocaína
or pasta base de cocaína, also often mentioned by the acro-
nym “PBC”, and other terms including “PBC seca”, “pasta
base”, “pasta”, “base”, together with many other “varia-
tions on the pasta theme” as Henman (2015) has aptly put
it. ese products are also reported to be smoked, both
mixed with tobacco or marijuana, or pure using home-made
pipes
15
(CICAD, 2019a; 2019b; 2016; 2014; JND, 2019;
2013; 2006; TNI, 2019; 2006; Moraes, 2015; Moraes et
al., 2015; Prieto et al., 2015; Sedronar, 2015; 2007; Arias
et al., 2014; Súarez et al., 2014; OUD, 2014; Ralón et al.,
2012; López-Hill et al., 2011; Pascale et al., 2010; Prieto
and Scorza, 2010; Capece, 2008; Míguez, 2008).
In turn, many sources dealing with drug markets elsewhere
in Latin America also use PBC (or the English acronym,
CBP), pasta base and similar variations to describe MCPs
available in other countries including Belize, Bolivia (pit-
illo), Brazil, Chile (mono), Colombia (basuco), Ecuador
(baserolo), Guatemala, Nicaragua, Panama, Peru (pay),
Paraguay (chespi) and Venezuela (CICAD, 2019a; 2019b;
2016; 2014; TNI, 2019; 2006; Henman, 2015; Duffau et
al., 2014; UNODC and OAS, 2014; Comunidad Andina,
2013; UNODC, 2013; Santis et al., 2007; Dormitzer et al.,
2004; Lizasoain et al., 2002; Dávila et al., 2001; Castaño,
2000; Jeri, 1984).
erefore, it appears that ‘paco’, ‘pasta base’, ‘PBC’ (CBP),
and the other “variations on the pasta theme” including
‘basuco’, are all equivalent terms used in order to describe
the various MCPs available on different Latin American
consumer markets.
Furthermore, the names suggest that all these consumer
products consist in coca paste (PBC) of varying cocaine
alkaloid concentrations and adulterant contents (eg.
UNODC, 2013). However, except in the case of crack
[SA], crack [BR] and merla in Brazil, no description of
how these products are prepared has been found in the
literature. Importantly, no forensic evidence has been found
in the literature confirming that the products thus named
15 The UNODC (2013, p. 54) provides a detailed description of the prep-
aration and smoking of a mixture of PBC and tobacco in a cigarette in
Peru. Photographs of different home-made pipes used in Colombia and
Argentina can be seen in the reports of the Transnational Institute (TNI,
2019) and Sedronar (2015).
are not prepared from substances other than coca paste
(PBC), such as cocaine base for instance. On the contrary,
there are indications that in Peru, several types of ‘PBC’
are available to users, one of which is called PBC lavada
and is described as a product ready for use in order to
manufacture cocaine hydrochloride (UNODC, 2013).
Similarly, Henman (2015) reports that several grades of
“pasta lavada” are available on the consumer market in
Lima. is is likely to be cocaine base.
Similarly, no evidence has been found that would exclude
the possibility that some of these products could be crack
[SA] (Colley and Casale, 2015), crack [BR] or merla
as described in Brazil (Zacca et al., 2014; Neves, 2013;
Medeiros et al. 2009)
16
. Yet, forensic studies in Brazil have
shown that crack [BR] and merla may be prepared alter-
natively from coca paste (PBC) or cocaine base, and there
is evidence indicating that “melted cocaine”, coca paste
(PBC) and cocaine base are smuggled from cocaine produc-
ing countries into Brazil. And it would be very surprising
if this were not also the case in other countries both near
(Argentina, Paraguay, Uruguay, Venezuela) and further
away (Belize, Chile, Ecuador, Guatemala, Nicaragua,
Panama) from Brazil, especially since most share borders
with Bolivia, Colombia and/or Peru.
In summary, it is likely that the terms ‘paco’, ‘pasta base’,
‘PBC’, etc., as used in many Latin American countries are,
like ‘basuco’ in Colombia, street names describing a range
of different MCPs. Although there is not enough forensic
evidence to draw definite conclusions, it is likely that these
terms as they are used in the literature cover in reality the
following consumer products (see Figure 2):
•
Coca paste (PBC), dried or damp, sometimes adulter-
ated with caffeine and phenacetin;
• Cocaine base, dried or damp, sometimes adulterated
with caffeine and phenacetin;
• Crack [BR] and crack [SA], as described in Brazil and
in Bolivia, Colombia and Peru, sometimes adulterated
with caffeine and phenacetin.
In addition, merla, as described in Brazil, and crack [FCP]
prepared from cocaine hydrochloride (see section of FCPs
below) may also be sold under different names or prepared
by users in Latin America.
16 Use of “crack” is reported by the Inter-American Drug Abuse Control
Commission in its last report on drug use in the Americas to occur in
8 South and Central American countries, in 4 Caribbean countries,
in Mexico and in the United States (CICAD, 2019a). However, since
CICAD (2019a) does not provide a precise definition of “crack”, it is
probable that this covers crack [FCP] prepared from cocaine hydro-
chloride and crack [SA] prepared from coca paste (PBC) or cocaine
base. The CICAD (2019a) report does not include information on
drug use in Brazil.
COCAINE INSIGHTS

23
The cocaine freebase consumer
products (FCPs)
Two FCPs have been identified: freebase and crack [FCP],
and both are primarily destined to be smoked, although
some users inject them. Both are prepared by subjecting
cocaine hydrochloride to relatively straightforward chemi-
cal processes using a weak base in order to transform the
cocaine hydrochloride salt into a base form that has been
freed of hydrochloric acid, hence the name “freebase”. e
main difference lies in the final stages of the processes which,
in the case of freebase, involve an additional extraction step
by means of an organic solvent (e.g. diethyl ether), which
results in the elimination of certain types of impurities.
ese processes mentioned above are sometimes referred
to as “freebasing” in English (Freye, 2009; OGD, 1996;
Bean, 1993), although the same term has also been applied
to the smoking of cocaine in freebase form (Gootenberg,
2008; Karch, 2008; Castaño, 2000; Farrar and Learns,
1989; Manschrek et al., 1988). In Latin American Span-
ish, transforming the cocaine hydrochloride salt into a base
form is sometimes colloquially referred to as “patraseo” or
“patraceado” meaning literally “turning back” or “send-
ing back” (TNI, 2019; CICAD, 2016a; UNODC 2013;
Molina, 2014; Castaño, 2000).
As in the case of the MCPs reviewed above, much of the
data and recent information available about the FCPs
appear to be characterised by ambiguity, lack of precision
and problematic availability. A difficulty arises out of the
fact that some datasets and reports do not discriminate
between the hydrochloride and freebase forms, conflating
both under the same heading of “cocaine” (EMCDDA,
2019b; SAMSHA, 2019; DEA, 2019; 2015), while others
group cocaine hydrochloride, FCPs and MCPs together
(UNODC, 2019b). In addition, in spite of notable excep-
tions (CICAD, 2016a; UNODC, 2013; Colussi-Mas et
al., 2003; Castaño, 2000; Perez-Reyes et al., 1982; Siegel,
1982), the literature infrequently differentiates between
crack [FCP] and freebase since they are chemically the same
form of the drug, and some conflate MCPs and FCPs as
they all are types of smokable cocaine.
FCPs users themselves may also confuse products and/
or altogether misconceive what they actually contain,
with a probable impact on the data collected by inter-
national organisations. In Paris, for instance, where most
crack [FCP] use in mainland France is concentrated, users
view crack [FCP] as a “dirty” drug made with sodium
bicarbonate and waste product from the cocaine manu-
facturing process. And they are convinced, erroneously,
that what they call “freebase” is a pure product because
it is made with cocaine hydrochloride and ammonia (see
below section on Freebase). A compounding factor is that
CHEMICAL
PROCESS
OPTIONAL
ADULTERATION
OPTIONAL
ADULTERATION
Hydro-
chloride-
based
powders
Crack [FCP]
Freebase
Volume 2

24
Preparation of crack [FCP]:
the misconception of using
ammonia
In Paris in 1993, the French monitoring centre
for drugs and drug addictions (OFDT) carried
out a study in which it tested the purity of a
purchased sample of cocaine hydrochloride
and of a set each of crack [FCP] and so-called
“freebase”. Each set was prepared, for the pur-
poses of the study, from the same purchased
sample of cocaine hydrochloride by different
users with some using sodium bicarbonate
and others ammonia. The study found that both
ammonia and sodium bicarbonate resulted in
a somewhat purer product than the starting
cocaine hydrochloride material and that similar
purities were observed regardless of whether
sodium bicarbonate or ammonia was used.
It also found that most of the cutting agents
present in the starting material were present
in the end product, proving that users’ percep-
tions were disconnected from the reality of the
products (OFDT, 2013) (see below section on
Freebase). A similar disconnection between
users’ perceptions of product quality and the
actual composition of products available on the
French market as revealed by chemical analysis
was also encountered in the case of heroin
(Dujourdy and Besacier, 2010).
in Paris crack [FCP] tends to be purchased ready-made
from dealers, while “freebase” is often home-made by its
users, who enjoy a certain prestige among their peers as
a result. e upshot is that at least some FCP users in
France are confident that what they inhale is freebase, and
they neither view nor report themselves as crack [FCP]
users but as cocaine users when they seek treatment or
participate in surveys. Much of their reluctance to view
themselves as crack [FCP] users has to do with the negative
image of crack [FCP] due to its perceived negative effects
on health and socioeconomic status (Reynaud-Maurupt,
2012). In contrast, freebase enjoys a much more positive
image (OFDT, 2018; 2013; Dujourdy et al, 2010; Freye,
2009; O’Rourke, 1991; Perry, 1980). Although it has not
been possible to identify evidence in other countries, it is
probable that similar misrepresentations of the reality also
occur outside mainland France.
One of the consequences of the above is that there appears
to be no specific data at all on the prevalence of freebase
use anywhere in the world at present, since much of the
recent literature and datasets refer to crack [FCP] or to
MCPs. And even when papers and reports explicitly men-
tion “cocaine freebase” it is often difficult to tell if they
mean freebase as it is defined here or crack [FCP] (Jekel et
al., 1994; Gold et al., 1985), or something else altogether
(Romo-Avilés et al., 2015).
ere is little doubt that crack [FCP] is more prevalent on
global drug consumer markets than freebase, and it has
recently been argued that preparing and smoking freebase
is an outdated, arcane practice falling into disuse (TNI,
2019). Yet this may not be the case everywhere and for every
category of users, and use of freebase may go unreported
in some markets such as the United States (Reuter and
Caulkins, 2004)
17
and Europe (EMCDDA and Europol,
2019, Pawlik and Mahler, 2011). In Europe, EMCDDA
data indicate that out of an estimated total of about
73 000 people entering treatment for cocaine problems in
2017, 15%, or about 11 000 people, sought treatment for
problems related to use of crack [FCP], which is typically
smoked. However, smoking/inhaling was reported as a route
of administration by a larger proportion of those entering
treatment for cocaine problems that year: a sizeable 26%
of the total, or about 19 000 users (EMCDDA, 2019b).
is could suggest that about 11%, or 8 000 people, of all
cocaine treatment entrants in Europe smoked or inhaled
cocaine but did not use crack [FCP], opening the possibil-
ity that at least some of them used freebase, or perhaps, as
in the case of the Paris users mentioned above, what they
(mis)conceived as “freebase”. At any rate, this suggests that
the number of users of FCPs in Europe is underestimated,
as it probably also is in other world regions. e obvious
conclusion is that more precise monitoring of the cocaine
market is needed.
Whatever the case, the literature analysed here suggests that
it is helpful for the purpose of understanding the phenom-
enon to distinguish freebase and crack [FCP] because they
are produced using different techniques and chemicals,
albeit from the same starting material (TNI, 2019; De
Souza, 2014; Neves, 2013; UNODC, 2013; Ribeiro, 2012;
Freye, 2009; Bono, 2008; Gootenberg, 2008; Blickman,
2006; Colussi-Mas et al., 2003; EMCDDA, 2001; Castaño,
2000; WHO and UNICRI, 1995; WHO, 1994). e key
difference is how the cocaine is recovered from the solution
in which it has been reacted with a weak base, and not
whether the weak base used is ammonia or sodium bicar-
bonate as it is sometimes thought (OFDT 2013; 2018). In
the case of freebase, the drug is recovered by liquid-liquid
extraction using an organic solvent, typically diethyl-ether,
which also purifies it. In the case of crack [FCP], little or
no purification is involved as the cocaine base is recovered
manually from the solution. Extraction with an organic
solvent will typically result in a purer end product than
the other method, but because it involves a flammable
solvent, it is much more dangerous (Freye, 2009; Bono,
2008; Colussi-Mas et al., 2003; WHO, 1994).
17 In a paper about the US markets for heroin and cocaine, Reuter and
Caulkins note that “cocaine base” appears “most commonly in the form
of ‘crack’” thereby suggesting that other forms such as freebase can also
be found (Reuter and Caulkins, 2004).
COCAINE INSIGHTS

25
In the 1970s and 1980s demand for cocaine increased dra-
matically in the United States (Gootenberg, 2008), and
cocaine powder was often viewed as a “soft” if relatively
expensive drug associated with wealth, success and the
artistic milieu. Particularly in California, several books
about cocaine were published at that time, some in expen-
sive, “coffee-table” formats. Titles included e Pleasures of
Cocaine (Gottlieb, 1976) and the Cocaine Consumer Hand-
book (Lee, 1976), and contents combined text with glossy
photographs and Art Deco drawings. ese publications
purported to teach readers about cocaine, its nature and
history and the various ways in which it could be used,
including smoking through pipes. Some even gave advice
for buying and dealing cocaine. Most also warned about
possible “undesirable side-effects” of cocaine use, many of
which were attributed to cutting agents. e books often
included chapters on how to purify cocaine powders, that
is, how to detect and eliminate cutting agents, notably by
subjecting the product to simple chemical processes using
ammonia and ether, for instance (Gottlieb, 1976).
As the interest in cocaine grew and more people starting
experimenting with the drug, a subgroup of freebase users,
essentially smokers, developed. At first, it seems that making
freebase was primarily presented as a method allowing users
to obtain a purer product than the one they had bought
from their dealers. Indeed, freebase was most frequently
“cooked” by those who would smoke it and only very rarely
bought from third parties (Siegel, 1982).
Crack [FCP]
Making crack [FCP] is not a sophisticated process and
requires no background in chemistry or specialised equip-
ment. It is achievable by using chemical ingredients available
in supermarkets and “do-it-yourself ” stores and utensils
present in any household, for instance spoons, pots and
pans, together with a source of heat like a cigarette lighter
or a stove. Unlike freebase, little physical risk is involved in
“cooking” crack [FCP] (see below). e relative ease and
safety of its manufacturing probably explains why crack
[FCP] has become a commercial product sold by dealers on
many drug markets in Europe, the Americas and elsewhere.
Crack [FCP] is prepared by dissolving cocaine hydrochloride
in water, then mixing a weak base such as sodium bicarbon-
ate (NaHCO
3
) or ammonia (NH
3
) in the solution. is is
then boiled until all precipitated cocaine base melts into
an oily layer, which occurs fairly rapidly. As the solution
becomes colder, the cocaine base oil solidifies at the bottom
of the recipient and is recovered with a tool, for instance the
point of a knife. e water is discarded. e cocaine base
can then be cut into smaller pieces if necessary and dried in
a microwave oven or under lamps, or even with a cloth or a
piece of kitchen roll in the case of small amounts.
Crack [FCP] made using this method will usually contain
most if not all of the impurities, diluents and adulterants
present in the starting material, albeit sometimes in lesser
amounts (Colley and Casale, 2014; Gostič et al., 2009;
Bono, 2008; Bean, 1993; Shannon, 1988; Siegel, 1982).
Commercial crack [FCP] manufacturers (as opposed to
user-manufacturers) may further dilute and/or adulter-
ate cocaine hydrochloride before processing it into crack
[FCP] (CICAD, 2019b). When more sodium bicarbonate
or ammonia than necessary is used for the preparation,
as is often the case in practice, residues of these sub-
stances will be present in the final product. While sodium
bicarbonate is unlikely to cause injury when inhaled,
ammonia is acutely toxic and will damage the lips, mouth,
windpipe and lungs if it has not been thoroughly washed
and dried off before the crack [FCP] is smoked. For this
reason, many harm-reduction organisations advise users
to prepare crack [FCP] with sodium bicarbonate and not
with ammonia.
Freebase: brief historical overview
Cocaine freebase became a fashionable product among
some groups of cocaine users in the United States start-
ing in the 1970s, approximately at the same time as the
smoking of MCPs began to be identified as a problem
in Peru (Jeri, 1984), but about 10 years before the emer-
gence of the “crack [FCP] epidemic” in the United States.
Although the information sources reviewed here address
freebase in the United States only, it is likely that the
product was also known and used in other regions includ-
ing Europe at that period.
© Jake Lyell / Alamy Stock Photo
Volume 2

26
In the late 1970s, books focusing exclusively on freebase
were published, promoting its effects as highly pleasurable
and listing several preparation methods (e.g., Anvil, 1979).
Kits for the small-scale preparation of freebase were sold
in drug paraphernalia shops or by mail order. ese com-
mercially available kits included basic equipment and small
amounts of chemicals like sodium bicarbonate, ammonia,
sodium hydroxide and ether. Five such kits and 5 freebase
preparation methods (including one involving the prepara-
tion of what we now call crack [FCP]) were evaluated in a
scientific study carried out by the University of Southern
California in the early 1980s (Siegel, 1982).
Like cocaine, or perhaps even more so since it was reserved
for discerning users of “pure” cocaine, freebase was sur-
rounded by an aura of prestige and has been described as
“the top-of-the-line model of the Cadillac of drugs” (Perry,
1980) and “the couture version of crack” (O’Rourke, 1991).
Many American freebase users were apparently well-off
individuals (Perry, 1980). However, accidents occurred due
to the use of the highly flammable ether in freebase prepa-
ration, and some users reported to hospitals with serious
burns. In a frequently mentioned episode, the American
comedian and actor Richard Pryor suffered severe burns
after accidentally igniting the rum he used instead of water
in his water-pipe during a 3-day freebase-smoking session
in his Los Angeles mansion in 1980.
By the early 1980s, it had become apparent that the smok-
ing of freebase could also be a source of significant health
problems, especially because freebase use was difficult
to control and users described engaging in compulsive
freebase smoking binge sessions that could last for days.
Freebase smoking began to be viewed as a medical prob-
lem as more users were seeking help and some scientists
launched into clinical studies (Anonymous, 1982; Perez-
Reyes, 1982). In the late 1980s, most media and scientific
attention became focused on another, cheaper product
where cocaine is in freebase form, crack [FCP], partic-
ularly on its reported connections with poverty, social
problems and crime (Goldstein et al., 1988), and freebase
subsided into the background.
Freebase preparation and purity
issues
Although the preparation of freebase is a little more com-
plex and much riskier than that of crack [FCP], it too can
be achieved without special chemistry skills, material or
chemicals. Making freebase requires dissolving cocaine
hydrochloride in water and adding a weak base (such as
sodium bicarbonate or ammonia). en liquid-liquid
extraction is performed by adding diethyl-ether (C
2
H
5
)
2
O,
or another volatile organic solvent, to the solution and by
stirring or shaking it. is causes the solution to separate
into 2 layers with the ether layer on top. e ether layer is
removed and transferred to another receptacle where it is
evaporated. e aqueous bottom layer is discarded. After
evaporation of the ether, solid crystals remain, looking like
small rocks or lumps.
Because the freebase recovery process, i.e. extraction with
a solvent, also is a purification process, preparing freebase
will often result in a purer form of cocaine than in the
cocaine hydrochloride powders used as starting material
or in crack [FCP]
18
. at said, as is the case with crack
[FCP], how pure the cocaine freebase is at the end of the
process depends largely on the purity of the cocaine hydro-
chloride used as starting material, and to some extent on
the organic solvent used. Indeed, extraction with ether will
cause all water-soluble substances to be captured in the
aqueous bottom layer, including some of the impurities and
common diluents (e.g. sugars such as mannitol, glucose,
lactose, sorbitol, etc.) present in the starting material, as
well as possible ammonia and sodium bicarbonate residue.
But many adulterants frequently found in cocaine hydro-
chloride powders, including, PTHIT substances including
levamisole, caffeine, diltiazem, hydroxyzine, phenacetin,
benzocaine, lidocaine, procaine, etc. (see section on adul-
terants below), will be entirely or partially captured in base
form in the ether layer, and after evaporation of the ether,
they will be part of the rock-like solids together with cocaine
freebase, and their vapours will be inhaled by users (Mallette
et al., 2013; UNODC, 2012; Pawlik and Mahler, 2011;
Freye, 2009; Gostič et al., 2009; Bono, 2008; Shannon,
1988; Siegel, 1982). As a result, freebase will rarely be pure
cocaine but simply purer cocaine.
e dangers associated with this method are due to the use of
an organic solvent, typically diethyl-ether, a highly flammable
chemical that will ignite if subjected to heat or a flame. us,
severe burns may result during the preparation of freebase,
or when smoking it if it contains residual or larger amounts
of ether (Freye, 2009; Bono, 2008; Siegel, 1982).
18 Liquid-liquid extraction with ether is one of the methods used to
purify cocaine base made from coca paste (Casale and Klein, 1993;
Schlesinger, 1985).
COCAINE INSIGHTS

27
Bioavailability of cocaine through smoking of crack [FCP] and freebase
Although freebase and crack [FCP] will often be
purer than the starting cocaine hydrochloride mate-
rial, this does not necessarily mean that smoking
will make cocaine bioavailable to the user, since a
considerable proportion of the drug will frequently
be trapped in the smoking device and in the respi-
ratory tract of users and will not reach the lungs
(and hence nor the bloodstream) (ElSohly et al.,
1991; Perez-Reyes et al., 1982). For instance, it
has been shown that smoking freebase or crack
[FCP] in a tobacco or marijuana cigarette can result
in a significantly lower bioavailability of the drug
than smoking through a water-pipe (Siegel, 1982).
In addition, the presence of adulterants can
drastically decrease the amount of cocaine actu-
ally entering the lungs. A study has shown that
cocaine base vapours decompose in the presence
of paracetamol, reducing the amount of cocaine
inhaled by the user. The proportion of the cocaine
that remains available to produce effects depends
on how much paracetamol is present. In a mix-
ture consisting of equal parts of cocaine base and
paracetamol about 97% of the cocaine is reported
to be destroyed; in a mixture containing just 10% of
paracetamol some 70% of the cocaine is reported
destroyed (Gostic et al., 2009).
markets, according to the information available. ere
is very little specific information on non-powder forms
of cocaine hydrochloride, such as solids, or on powdered
cocaine base (Dujourdy et al., 2010), and both appear to
be considerably rarer than cocaine hydrochloride powders.
As a result, and although it is reported to be practically
impossible to visually distinguish powders containing
cocaine hydrochloride from those containing cocaine base
(Dujourdy et al., 2010), use of the term “powder cocaine”
has become widespread as a practical, though imprecise
(King, 1997), way of distinguishing consumer products
containing cocaine hydrochloride from those containing
cocaine base and especially “crack cocaine” (or crack [FCP]
as it is called in this report) (CICAD, 2019a; DEA, 2019;
EMCDDA, 2019b; 2018a; UNODC, 2019b; Fischer et
al., 2016; NIDA, 2016; Shearer et al. 2005). is section
is based on the information that is available and therefore it
focuses exclusively on cocaine powders and, unless specified,
assumes that all contain cocaine hydrochloride.
Strictly speaking, the powders sold as cocaine to consumers
around the world are practically never 100% pure cocaine
hydrochloride. In other words, all powders will contain
substances other than cocaine hydrochloride in greater or
lesser amounts (while some “fakes” will have no cocaine
at all). Cocaine hydrochloride powders are therefore best
understood as mixtures of a range of different substances
present in varying, unpredictable proportions.
Two categories of non-cocaine hydrochloride substances
are most commonly reported in the cocaine powders
seized around the world: impurities and cutting agents.
ese substances come from 3 different sources: the plant
material used to manufacture cocaine hydrochloride; the
cocaine manufacturing process; and the process of dilu-
tion and adulteration implemented by markets actors, i.e.
cocaine producers and traffickers (Schlesinger, 1985). Cut-
ting agents account for by far the largest proportion of
the non-cocaine material usually found in most cocaine
hydrochloride powders.
OPTIONAL
ADULTERATION
cocaine
hydrochloride
Hydro-
chloride-
based
powders
Cocaine hydrochloride: a mixed bag
of unpredictable powders
Powder is what comes to people’s minds when they think of
cocaine and powder is how cocaine hydrochloride is most
frequently made available to consumers on illicit global
Volume 2

28
Impurities
It should be noted that, in contrast to cutting agents, some
impurities will practically always be present in the cocaine
hydrochloride products made available to global consum-
ers (Casale and Klein, 1993; Soine, 1986)
19
. is is very
likely to include products that are sometimes reported to
contain “only cocaine” or even “pure cocaine” by drug-
checking services, which infrequently test cocaine samples
for impurities and rarely report on diluents (McDonald et
al., 2020; Payer et al., 2020; Brunt et al., 2016; Caudevilla
et al., 2016; Ventura et al., 2011; TEDI, n.d.).
Alkaloids
One of the families of impurities found in cocaine hydro-
chloride consumer products arise from the plant material.
ey consist in several natural compounds, that is, the alka-
loids present in coca leaves that have not, or not totally,
been eliminated during the cocaine manufacturing process,
for instance by oxidation with potassium permanganate.
Different species, varieties, and cultigens of the coca plant
will yield different ranges of alkaloidal impurities in cocaine
hydrochloride powders. Additional alkaloidal impurities may
be generated by the manufacturing process itself through the
chemical modification of cocaine and other coca alkaloids.
A third type of alkaloidal impurities may emerge from the
degradation of cocaine hydrochloride, cocaine base and coca
paste due to heat and humidity such as may occur when
these products are stored for a long period and/or in poor
conditions. Analysis of alkaloids present in cocaine samples
can be useful for determining the varieties of coca plants
used for cocaine manufacturing as well as some aspects of the
manufacturing process, for instance the extent of oxidation
of the cocaine or the types of solvent used.
Cis-cinnamoylcocaine, trans-cinnamoylcocaine and tropa-
cocaine appear to be the alkaloids present in larger amounts
in most illicit cocaine hydrochloride powders (Zacca et al.,
2014; Comunidad Andina, 2013; Casale et al., 2008b;
By et al., 1988; Soine, 1986). Other commonly found
alkaloidal impurities include benzoylecgonine, ecgonine,
methylecgonine (ecgonine methyl ester), hydroxycocaines,
norcocaine, trimethoxycocaine, truxillines and others.
Concentrations of alkaloidal impurities in cocaine hydro-
chloride samples are reported to range from tiny amounts to
a not negligible 10% of the total (Cui et al., 2019; Mallette
et al. 2018; Maldaner et al., 2016; Monfreda et al., 2015;
Stride Nielsen et al., 2016; Botelho et al. 2014; Casale et
al., 2014; Mallette and Casale, 2014; Zacca et al., 2014;
Comunidad Andina, 2013; Casale et al., 2007; Fucci, 2007;
Moore and Casale, 1994; Casale and Klein, 1993; Gómez
and Rodríguez, 1989; Soine, 1986; Schlesinger, 1985).
19 Casale and Klein (1993) note that even pharmaceutical cocaine is not
100% pure.
Solvents and other chemicals
e preparation of cocaine hydrochloride from coca leaf
will be an additional source of some of the non-cocaine
material found in products sold to the end consumer.
Although a series of other residues including acids and
bases are also found in consumer cocaine powders (Magal-
hães et al., 2013), solvents are the most frequently reported
residues of illicit cocaine manufacturing. Most solvents
are highly toxic substances, and even if they are usually
present in residual amounts in cocaine powders, repeated
exposure in frequent users may lead to a series of negative
health outcomes (Garzón et al. 2009).
Solvents are used at several stages of the manufacturing
process and small amounts are almost always occluded in
the crystals of illicit cocaine hydrochloride powders sold to
consumers. Solvents encountered in cocaine hydrochloride
products may also have been added after the manufacturing
process, for instance as impurities in cutting agents (Morello
and Meyers, 1995). A wide range of residual solvents have
been found in cocaine hydrochloride samples over the years
since different solvents are used in different combinations at
different times by illicit cocaine manufacturers in Bolivia,
Colombia and Peru. For example, a study of 65 samples
taken from several 1-kilo bricks of cocaine hydrochloride
seized in Colombia identified a total of 28 different solvents
(Garzón et al. 2009).
A wide variety of solvents were also detected in cocaine
hydrochloride samples analysed in Europe and in the
United States since the early 2000s. For example, a forensic
study in Denmark in the mid-2010s identified the pres-
ence of a total of 13 different solvents in just 5 samples
of seized cocaine hydrochloride, while individual samples
each contained between 4 and 9 different solvents (Stride
Nielsen et al., 2016). Fifteen years earlier an Italian study
had identified a total of 32 different solvents in 47 samples
of cocaine hydrochloride (Chiarotti et al., 2002).
Solvent combinations change over time as illicit manufac-
turing processes evolve constantly in response to changes
in manufacturing methods, to new opportunities for
procuring specific solvents and to precursor control and
enforcement measures implemented in cocaine producing
countries and in source countries for cocaine chemicals.
A recent example of such shifts is an increase in the use of
acetate solvents other than ethyl acetate when converting
cocaine base to cocaine hydrochloride (INCB, 2020; 2019).
Solvents commonly found in cocaine hydrochloride con-
sumer products include acetone, benzene, chloroform,
diethyl-ether, ethanol, ethyl acetate, hexane, isobutanol,
isopropylacetate, methylene chloride, metyl etyl ketone
(MEK), n-propyl acetate, toluene, etc. (Stride Nielsen et al.,
2017; 2016; Monfreda et al., 2015; Colley and Casale, 2014;
Dujourdy et al., 2010; Dujourdy and Besacier, 2008; Casale
et al., 2008a; Chiarotti et al., 2002; Schlesinger, 1985).
COCAINE INSIGHTS

29
Forensic analysis of changes in the solvent profiles of cocaine
hydrochloride samples can also be useful in order to identify
changes in illicit cocaine manufacturing methods. A recent
illustration is the identification of a trend in which illicit
cocaine manufacturers now process multi-kilogram batches
of cocaine base into cocaine hydrochloride, whereas in the
past cocaine hydrochloride was produced 1 kilogram at a
time (Mallette et al. 2018).
Cocaine base alongside cocaine
hydrochloride
In addition to the impurities commonly found in cocaine
powders, some powders also appear to contain cocaine base
alongside cocaine hydrochloride. e presence of cocaine base
in cocaine powders is reported to be unintended and due
to incomplete, i.e. faulty, crystallization of cocaine base into
cocaine hydrochloride in the illicit manufacture process. e
analysis of about 12,500 samples of cocaine powders seized
in France has shown that powders containing both cocaine
base and cocaine hydrochloride were seized every year over
a 20-year period (1990-2009). In some years, such mixtures
represented almost 20% of all cocaine powders seized and
analysed in the country (Dujourdy et al., 2010; Dujourdy
and Besacier, 2008). Similar base-hydrochloride mixtures were
found in Brazil (Zacca et al., 2014), and in a small number
of samples of cocaine seized in Canada in 2019 (CSP, 2020).
Although sources describing similar situations in other
countries have not been found, it is likely that powders
containing mixtures of cocaine base and cocaine hydro-
chloride exist on global consumer markets on a larger scale
than the available data suggest. is information gap could
be due, at least partly, to the fact that specific tests (e.g.
infra-red spectrometry) required to confirm the presence of
cocaine hydrochloride in samples are not always performed
in forensic laboratories (King, 1997).
Cutting agents: diluents and
adulterants
It is well-known that cutting agents, also known as addi-
tives, cheaper than cocaine are commonly added to cocaine
hydrochloride powders by market actors along the illicit
distribution chain mainly in order to increase profits by
increasing product volume. However, some cutting agents
are likely to serve additional purposes as well (see below).
Unlike precursors and essential chemicals, cutting agents
are typically not subject to international control, although
some may be subject to controls under national legislation,
including health and/or food legislation.
An obvious prerequisite for a substance to be used as a cutting
agent in cocaine powders is that it must physically resemble
the substance sought by buyers so that they do not become
aware of the fact that they are obtaining a diluted or adul-
terated product. erefore, most cocaine cutting agents are
white or off-white powders.
Some cutting agents, specifically adulterants, are added
at the last stage (crystallization) of the cocaine hydro-
chloride manufacturing process at buyers’ request; buyers
are reported to supply the adulterants and to specify
the amounts to be added to the cocaine hydrochloride
(SIMCI, 2019b; INCB, 2018; 2017). However, cocaine
is also frequently cut at all subsequent stages (Morelato
et al., 2019; Broséus et al., 2016; Magalhães et al., 2013;
Caulkins and Reuter, 1998). As a result, cocaine hydro-
chloride consumer products are likely to be among the
powder drugs most subject to dilution and adulteration,
at least in the Americas and Europe (CICAD, 2019a;
Broséus et al., 2016; Karch and Drummer, 2015; TEDI,
n.d.). For instance, there is evidence suggesting that
cocaine hydrochloride powders are more heavily and
more frequently diluted than heroin powders in Europe.
Wider varieties of diluents and adulterants were found
in larger amounts and in more samples of cocaine hydro-
chloride consumer products than in heroin products in
comparative or comparable studies involving thousands
or hundreds of cocaine hydrochloride samples (Morelato
et al., 2019; Broséus et al., 2015b; 2016; Schneider and
Meys, 2011; Dujourdy and Besacier, 2010; Dujourdy et
al., 2010; Andreasen et al., 2009).
Two categories of cutting agents may be distinguished:
diluents and adulterants. Diluents are inert, pharmacologi-
cally inactive substances. Many diluents found in cocaine
products are routinely used in the food industry (e.g. sugars,
starches, bicarbonates), and like the other products used
to dilute cocaine, they can be purchased with relative ease
and at comparatively low prices.
Adulterants are pharmacologically active substances, and
they tend to be more expensive and harder to procure since
they may be subject to more national controls, as many
of them are pharmaceutical drugs. Importantly, several
of the adulterants frequently found in cocaine products
are harmful substances that amplify the toxicologic effects
of cocaine. In fact, some adulterants may be more harm-
ful than cocaine itself (Knuth et al., 2018; Brunt et al.,
2017; Hammond and Craven 2017; Martelo et al., 2017;
Solomon and Hayes, 2017; Busardò et al., 2016; CICAD,
2016a; Indorato et al., 2016; Pawlik et al. 2015; Barbera
et al., 2013; Comunidad Andina, 2013; Pilgrim et al.,
2013; Kachiu et al., 2012; Karch et al., 2012; Larocque and
Hoffman, 2012; Buchanan et al., 2010; Cole et al., 2010;
Knowles et al., 2009; Raymon, and Isenschmid, 2009;
Fucci, 2004; Karch, 1996; Shesser et al., 1991; Curini et
al., 1989; Shannon, 1988).
Volume 2

30
Diluents
Diluents, sometimes called bulking agents, fillers or excipi-
ents, are pharmacologically inert substances whose only
function, other than inconspicuously bulking up cocaine
products, can be aesthetic, that is, giving powders an aspect
and consistency that will be appealing to consumers. Less
information about diluents is available in the scientific
literature than about adulterants, which may be due to
the fact that most diluents are viewed as posing less health
risks to cocaine consumers than do many adulterants (Solo-
mon and Hayes, 2017). However, high concentrations of
diluents in cocaine products can lead to negative health
outcomes (TEDI, n.d.), for instance maize starch and talc
when cocaine is injected (Shannon, 1988).
A wide variety of diluents have been found in samples of
cocaine hydrochloride consumer products. e evidence
indicates that dilution of cocaine hydrochloride occurs in
producing, transit and consumer countries, but perhaps more
so in the latter two (Morelato et al., 2019; Sant’Ana et al.
2019; SIMCI, 2019b; Zacca et al., 2014; Magalhães et al.,
2013; Cunningham et al., 2010; Shannon, 1988). Diluents
found in cocaine hydrochloride samples in Brazil, Canada,
Chile, Europe, Morocco and the United States since the mid-
1980s include sugars (dextrose, fructose, glucose, inositol,
lactose, sucrose, maltose, mannitol, sorbitol, etc.) and other
substances such as ascorbic, boric, citric and tartaric acids,
carbonates and bicarbonates (e.g. calcium carbonate), sodium
chloride (table salt), potato, wheat and maize starches, sul-
phates (e.g. aluminium sulphate, plaster), talc and other
silicates, etc. (Duffau et al., 2020; CICAD, 2019b; Morelato
et al., 2019; Sant’Ana et al., 2019; Da Silva, 2018; Solimini
et al., 2017; Stambouli and El Bouri, 2017; Maldaner et al,
2016; Broséus et al., 2015b; Monfreda et al., 2015; Duffau
et al., 2014 ; Magalhães et al., 2013; Neves, 2013; Brunt,
2012; Cole et al., 2011; Cole et al., 2010; Dujourdy et
al., 2010; Andreasen et al., 2009; Neves and Nunes, 2008;
UNODC, 2005 ; Gonçalves de Carvalho and Mídio, 2003;
Fucci and De Giovanni, 1998; King, 1997; Curini et al.,
1989; Gómez and Rodríguez, 1989; Shannon, 1988; Janzen,
2013; Cunningham et al., 1984; Siegel, 1982).
Trends in use of diluents are difficult to identify due to
variations in both the diluents used by market actors in dif-
ferent settings at different times and the methodologies and
scopes (periods covered and sample numbers) of the relatively
few recent studies from which diluent information can be
retrieved. at said, the literature reviewed here tentatively
suggests that between the 1980s and the mid-2010s the
cocaine diluents most frequently found in South America
were carbonates and bicarbonates (Duffau, 2020; Sant’Ana
et al., 2019; Duffau et al., 2014; Magalhães et al., 2013; Ber-
nardo et al., 2003; Gonçalves de Carvalho and Mídio, 2003;
Morales-Vaca, 1984), whereas those most frequently used
in Europe were sugars (Morelato et al., 2019; Broséus et al.,
COCAINE INSIGHTS

31
2015a; Cole et al., 2011; Dujourdy et al., 2010; Andreasen
et al., 2009; Brunt et al., 2009; Decorte, 2001; Fucci, and
De Giovanni, 1998; King, 1997; Gómez and Rodríguez,
1989; BBC, n.d.).
Adulterants
Adulterants added to cocaine hydrochloride powders consist
of a wide range of pharmacologically active substances that
most frequently includes medicines, more rarely other illicit
drugs like amphetamine and methamphetamine (Payer et al.,
2020; Kudlacek et al., 2017; Cole et al., 2011; Brunt at al.,
2009; Gómez and Rodríguez, 1989), and even more rarely
new psychoactive substances (Payer et al., 2020; Kudlacek et
al., 2017; Vidal et al., 2014). Adulterants are usually costlier
and may be more difficult to procure than diluents, and as a
result they have long been suspected to serve purposes other
than simply bulking up cocaine products (Shannon, 1988).
A specific “grey” market for adulterants found in cocaine
and other drug products exists in Europe (EMCDDA and
Europol, 2016; ACMD, 2015; Broséus et al., 2015b; 2016;
Dujourdy and Besacier, 2010) and probably in other regions
such as the Americas.
Adulterants account for the largest proportion of the non-
cocaine hydrochloride material most frequently reported in
cocaine consumer products, and they can pose significant
additional health risks because of their individual proper-
ties and their interactions with cocaine or with one another
(Pawlik et al., 2015). Yet their presence in cocaine products
is not routinely monitored using standardized chemical
analysis methodologies at international or regional level
(CICAD, 2019b; Lociciro et al., 2008), although, according
to the INCB (2019), this would be possible under article 13
of the United Nations Convention against Illicit Traffic in
Narcotic Drugs and Psychotropic Substances of 1988. One
of the consequences is that this gap renders comparisons
across regions or countries, and over time, of the prevalent
cutting practices of market actors—for the purposes of
strategic analysis and public health interventions—both
more difficult to implement and less reliable (Broséus, et
al., 2016; Busardò et al., 2016; Barrio et al., 1997).
As is the case with diluents, the adulterant content of hydro-
chloride powders varies widely in terms of the number of
individual substances used, their different combinations,
and their concentration relative to cocaine. Adulterant con-
tents also change with time (Broséus, 2016), and the range
of adulterants found in cocaine hydrochloride samples has
increased since the 1980s in Europe and the Americas,
when mostly local anaesthetics were used, and especially
since the early- to mid-2000s (CICAD, 2019b; Broséus et
al., 2015a; 2015b; Brunt, 2012; Cole et al., 2010; Brunt et
al., 2009; Casale et al., 2008b). However, there are indica-
tions that adulterant concentrations in cocaine powders
have decreased recently in several European markets and
in the United States, where cocaine purity has increased
(DEA, 2019; EMCDDA and Europol, 2019; Verri et al.,
2019; Gómez and Rodríguez, 1989; Shannon, 1988).
e wide range and diversity of adulterants used to cut
cocaine products illustrates the complexity and dynamism
of the phenomenon of cocaine adulteration and of the
global cocaine market in general. us, a UNODC (2005)
manual for forensic laboratories has listed 30 substances
(including five controlled drugs) as frequently encountered
adulterants in cocaine products, whereas 38 (including two
controlled drugs) were listed in a paper published in the
United States in the early 1990s (Shesser et al. 1991), and
a report states that 50 different adulterants were found in
consumer cocaine powders seized in the United Kingdom
in the mid-2010s (ACMD, 2015). Nonetheless, according
to the literature reviewed for this report, the number of
different adulterants of cocaine hydrochloride consumer
products identified in individual studies reporting chemical
analysis results published over the past 30 years or so varies
roughly between 5 and 15 different substances.
Over the past 15 years, the most frequently encountered
adulterants in cocaine hydrochloride products have been
caffeine, diltiazem, hydroxyzine, levamisole and dexami-
sole, paracetamol and phenacetin as well as a range of local
anaesthetics led by lidocaine (sometimes called lignocaine)
but also including benzocaine, prilocaine, procaine and
tetracaine. e information available suggests that local
anaesthetics are the substances with the longest history of
use as cocaine adulterants, while levamisole (a substance
widely used in veterinary medicine) and phenacetin (an
analgesic) appear as those most frequently found in the last
10 to 15 years (Duffau et al., 2020; INCB, 2020; Payer
et al., 2020; CICAD, 2019b; Cui et al., 2019; Fiorentin
et al., 2019; INCB, 2019; SIMCI, 2019b; Bertol et al.,
2018; Da Silva et al., 2018; INCB, 2018; Villar, 2018;
Brunt et al., 2017; INCB, 2017; Stambouli and El Bouri,
2017; De Souza et al., 2016; Maldaner, 2016; Marcelo et
al., 2015; Broséus et al., 2015a; 2015b; Lapachinske et al.,
2015; Botelho et al., 2014; Eiden et al., 2014; Floriani et
al., 2014; Comunidad Andina, 2013; Casale et al., 2012;
Schneider and Meys, 2011; Ventura et al., 2011; Cole et al.,
2010; Dujourdy et al., 2010; Evrad et al., 2010; Andreasen
et al., 2009; Brunt et al., 2009; Maietti et al., 2009; Beh-
rman et al., 2008; McGill et al., 2008; Neves and Nunes,
2008; Kenyon et al., 2005; Fucci and De Giovanni, 1998;
Barrio et al., 1997; King, 1997; Gómez and Rodríguez,
1989; Shannon, 1988; Cunningham et al., 1984; Siegel,
1982; Anvil, 1979; Lee, 1976; BBC, n.d.; TEDI, n.d.).
Most of the adulterants found in cocaine hydrochloride
powders are pharmaceutical drugs, although in some
cases their use in human medicine has been discontin-
ued or restricted in many countries due to their adverse
effects (e.g. levamisole, phenacetin). e main adulter
-
ants of cocaine belong to the following pharmacologic or
therapeutic families, with some belonging to more than
one family: local anaesthetics, analgesics (pain killers),
Volume 2

32
Data and information sources about adulteration of cocaine powders
used in this report
A fairly large body of information about adulter-
ants in cocaine hydrochloride consumer products
available in several drug markets does exist in the
literature, but most published studies cannot be
said to be representative of national situations and
they often cover different time periods. The few
relatively recent studies reporting results of analysis
of thousands of samples seized over long periods
of time that could be viewed as a better reflection
of national or subnational situations and trends, are
necessarily limited to the timeframe and locations
they cover and they usually only take place once
(Villar et al., 2018; Broséus et al., 2015b; Comunidad
Andina, 2013; Dujourdy et al., 2010; Brunt et al.,
2009). Another limiting factor is that the vast major-
ity of the information found in published forensic
reports relates to illicit markets in Brazil, Canada,
several, mostly western, European countries and
the United States. These and other limitations com-
bine to make the evidence, and the image that can
be built from the literature, generally patchy, even
in the comparatively few markets where evidence
is available.
The majority of the publicly available information
on adulterants present in cocaine hydrochloride
consumer products reviewed in this report comes
from two sources:
-
Official forensic laboratories of national or
sub-national authorities reporting results of
chemical analysis of seized samples, which
make up the majority of the literature reviewed
here; and
- Drug checking services reporting information
on analysis performed on cocaine hydrochlo-
ride products submitted to them by users.
A limitation affecting most forensic studies comes
from their source material, that is, samples of
cocaine products seized by law enforcement
authorities, which often include cocaine exchanged
between market actors as well as consumer prod-
ucts, and are therefore not always focused neatly
on the latter. That said, the adulterants identified
in seizures of larger amounts of cocaine are more
than likely to also be found further down the chain,
at consumer level. Another limitation of many of
these studies is their lack of immediacy, that is,
they tend to be reflections of past states of affairs
and often feature results of analysis of samples
that were seized several years before publication.
A useful source in this respect is the regular analysis
of seizure samples, such as in the United States by
the Cocaine Signature Program, which has reported
data for several years on concentrations of phenyl-
tetrahydroimidazothiazole (PTHIT), i.e. levamisole,
its stereoisomer dexamisole, and combinations
thereof, in samples of wholesale cocaine seizures
carried out in the United States market (Mallette et
al., 2013; Casale et al. 2012; Casale et al., 2008b;
Valentino and Fuentecilla, 2005).
Publicly available reports of drug checking services
can also provide useful specific information on adul-
terants encountered in cocaine consumer products.
In addition, they tend to be more timely than foren-
sic studies, often reflecting present or very recent
situations. However, they are rarely representative
of national or even subnational situations. Another
limitation is that most such services exist only in
Canada, eight European countries and the United
States, and they are primarily active in nightlife set-
tings (Maghsoudi et al., 2020; McDonald, 2020;
Payer et al., 2020; EMCDDA, 2018b; Brunt, 2017;
Ventura et al., 2011; DrugsData, n.d.), thereby under-
representing or excluding other contexts in which
cocaine is used.
Some insight into adulterants in cocaine hydrochlo-
ride consumer products can also be gained from
studies analysing residue in syringes used to inject
drugs. However, injection appears to be much less
frequently used than other routes of administra-
tion for cocaine, while syringe residue analysis is
a relatively novel technique and there have been
few reports from a handful of European cities so
far (EMCDDA, 2019a; Néfau et al., 2015).
Data on adulterants from drug checking and syringe
residue analysis have been added recently to the
range of sources regularly used in the monitoring of
the drug market in the European Union (EMCDDA,
2019a; 2019b) and similar initiatives also exist in
Canada (Maghsoudi et al., 2020; Payer et al. 2020).
In future, this may help improve knowledge about
cocaine hydrochloride consumer products available
on some markets.
COCAINE INSIGHTS

33
anthelminthic (anti-worm), antihistamines (anti-allergy),
antipyretics (fever reducing), anxiolytics, sedatives, stim-
ulants (sometimes called “nootropics”) and vasodilators
(heart medication). Many cocaine adulterants pose sig-
nificant health risks, some of which are summarised in
Table 1 (CICAD, 2019b; Knuth et al., 2018; Brunt et al.,
2017; Hammond and Craven 2017; Martelo et al., 2017;
Busardò et al., 2016; Indorato et al., 2016; Broséus et al.,
2015a; Pawlik et al. 2015; Barbera et al., 2013; Comunidad
Andina, 2013; Pilgrim et al., 2013; Brunt, 2012 ; Larocque
and Hoffman, 2012; Karch et al., 2012; Cole et al., 2011;
Ventura et al., 2011; Buchanan et al., 2010; Cole et al.,
2010; Fucci, 2004; Karch, 1996; Shannon, 1988).
However, it must be stressed that the properties for which
these substances are or were used to treat patients may not
necessarily be those that are sought by the market actors
who add them to cocaine products. Which properties
do market actors seek then? According to the literature
reviewed here, for some adulterants their motivations seem
transparent but in several other cases, including harmful
substances, there are no certainties. In addition, although
the toxicity of specific cocaine adulterants is generally
known when they are used as medicines, often as tablets
administered orally, this may not be the case when they
are used in combination with cocaine or other adulterants
and/or via different routes of administration such as nasal
insufflation and smoking (Pawlik et al., 2015; Brunt et al.,
2009). us, the issue of cocaine adulteration and its effects
on health remains a partial knowledge gap.
Purity of cocaine hydrochloride
products and changes in the global
market
Although it is also a reflection of the efficiency of illicit
manufacturing facilities, cocaine purity appears almost
as an image in negative of dilution and adulteration, as
illustrated in Figure 3: the more cutting agents are mixed
into cocaine products, the less pure they are, and vice-versa.
Another aspect is worth noting: the impact of cocaine
adulteration at the source. Considering that levamisole
is known to be added primarily to Colombian cocaine
crystallization laboratories (SIMCI, 2019b), Figure 3 also
appears to indicate that, at least since 2010, adulteration at
the source has had a major impact on wholesale purity and,
more surprisingly, on retail purity of cocaine hydrochloride
products in a major destination market, that of the United
States. Indeed, it is often reported that dilution and adul-
teration occur primarily after cocaine has left production
facilities and especially at levels closer to the retail market
(Morelato et al., 2019; Broséus et. al, 2016; Cunningham
et al., 2010; Kilmer and Hoorens, 2010; Caulkins and
Reuter, 1998). While Figure 3 does not necessarily disprove
this, it may reflect an increased availability of cocaine in
the global cocaine market in the 2010s.
Other products
Although this appears to be rare, it is worth mentioning that
cocaine-containing products made to look like medicines
can occasionally be encountered on illicit markets. e
United States Drug Enforcement Administration (DEA)
recently reported that tablets and capsules containing
cocaine are sporadically seized in the United States. In the
2018-2019 period, some of the capsules seized contained
cocaine only, but in other instances cocaine was found in
tablets also containing other controlled substances such
as buprenorphine and alprazolam (Xanax). For instance,
in Massachusetts in January 2019, cocaine was found in
combination with alprazolam in a counterfeit prescription
2-milligram alprazolam tablet while similar tablets seized at
the same time were found to contain alprazolam combined
with fentanyl. e DEA does not specify the amounts of
cocaine found in each tablet/capsule or if the drug was
in base or hydrochloride form (DEA, 2019). As far as is
known, tablets or capsules containing cocaine have not
been reported anywhere else in the world in recent years.
It is difficult to ascertain how these products were destined
to be used based on the information reported by the DEA.
However, it can be doubted that they were meant to be
ingested orally since this route of administration would
result in the destruction of 60% to 70% of the cocaine
before it had produced any effect.
FIG. 3
Cocaine purity versus adulteration with
levamisole/dexamisole, United States,
2010 and 2018
Sources:
Purity: Office of National Drug Control Policy
Adulteration:
- 2010: DEA cocaine signature programme, data summarized in:
Casale, J., Colley, V. and LeGatt, D. (2012), “Determination of
Phenyltetrahydroimidazothiazole Enantiomers (Levamisole/Dex-
amisole) in Illicit Cocaine Seizures and in the Urine of Cocaine
Abusers via Chiral Capillary Gas Chromatography–Flame-Ion-
ization Detection: Clinical and Forensic Perspectives”, Journal of
Analytical Toxicology, vol. 36, n°2, March.
- 2018: Drug Enforcement Administration. January 2019 CSP
Report, DEA PRB 05-13-19-09, 2019.
Cocaine purity (left to right)
Percentage of seized cocaine bricks containing
levamisole/dexamisole (right to left)
Retail purity (upper axis)
Wholesale purity (upper axis)
Percentage of seized cocaine bricks examined by Cocaine Signature
Programme containing levamisole/dexamisole (lower axis)
2010
2018
2010
2018
0%
0%
15%
15%
30%
30%
45%
45%
60%
60%
75%
75%
90%
90%
Volume 2

34
The use of levamisole as an adulterant
Levamisole has been described as “the ideal cut-
ting agent” (Solomon and Hayes, 2017) and it is
certainly the cocaine adulterant on which most
information is available. This is undoubtedly due to
its toxicity and near ubiquitous presence in cocaine
samples tested around the world in the last 15
years, although most of the literature on levamisole
relates to cocaine markets in Europe and North
America. However, it should be noted that other
substances such as caffeine and phenacetin have
been used to adulterate cocaine products for a
longer period of time than levamisole and have
often been found in higher concentrations and
in more samples than levamisole (SIMCI, 2019b;
Comunidad Andina, 2013; Dujourdy et al., 2010;
Brunt et al., 2009; Gómez and Rodríguez, 1989).
Levamisole, an isomer of phenyltetrahydroimid-
azothiazole (PTHIT), was originally developed as
an anthelmintic medicine for humans and animals
in the mid-1960s (Solomon and Hayes, 2017). Use
of the drug in human medicine was subsequently
abandoned due to severe adverse effects. At pres-
ent, levamisole is widely used in many countries as
a veterinary medicine in order to get rid of worms
in caprine, bovine, ovine and porcine livestock.
Although levamisole is generally no longer used
in human medicine, it still has a very restricted
number of applications in some countries, for
instance as a kidney drug for children, as an adju-
vant in treatment of colon cancer or in treatment
of rheumatoid arthritis (Brunt et al., 2017; CICAD,
2016a; ANSM, 2014; Kachiu et al., 2012). It is worth
noting that residues of levamisole and other anthel-
mintic drugs used to treat livestock have been
identified in varying concentrations in cooked beef
and pork meats and their juices, and are therefore
also likely to be ingested by the non-cocaine-using,
meat-eating population (Cooper et al., 2011).
Levamisole is added to cocaine hydrochloride pow-
ders by producers in South America, essentially in
Colombia (SIMCI, 2019b; INCB, 2018; 2017; Comu-
nidad Andina, 2013; Casale et al., 2012; Garzón et
al. 2009; Casale et al. 2008b) but some may also
be added in other producing, transit or consumer
countries. It is possible that, initially, levamisole
was added to cocaine hydrochloride mainly in prod-
ucts destined to be exported out of South America,
possibly in mixtures containing other adulterants.
Soon after it was detected as an ingredient
in cocaine hydrochloride powders for the first
time in the United States in 2003 (Casale et al.,
2008b; Valentino and Fuentecilla, 2005), levami-
sole-adulterated cocaine hydrochloride was also
identified in Canada in 2004 (LeGatt et al., 2007)
and in Europe. It was detected for the first time in
France and in the Netherlands in 2004 (Dujourdy
et al, 2010; Brunt et al., 2009), in Luxembourg in
2006 (Schneider and Meys, 2011), “significantly
detected” for the first time in Switzerland in 2006
(Broséus et al., 2015b), and first reported in Italy
in 2007 (Fucci, 2007). In Morocco, it was detected
for the first time in 2009 (Stambouli and El Bouri,
2017).
In mid-2010, illicit cocaine manufacturers also
started adding tetramisole, alone or in combina-
tion with levamisole, as an adulterant in cocaine.
Tetramisole is a commercially available anthelmin-
tic made up of equal proportions of levamisole
and dexamisole, the other isomer of phenyltetra-
hydroimidazothiazole (PTHIT) (Casale et al., 2012).
By the end of the 2000s and up to the mid-to-
late-2010s, it is likely that levamisole, alone or
in combination with tetramisole, was present
in many, then most, cocaine hydrochloride pow-
ders sold in Australia (Pope et al., 2018), Europe
and North America. Starting in April 2009 it was
detected in more than 50% of the 1-kilo cocaine
bricks seized in the United States and analysed
by the DEA’s Cocaine Signature Program (CSP)
(Casale et al., 2012). The same year, about 45%
of the cocaine hydrochloride products seized and
analysed in France contained levamisole (Dujourdy
et al., 2010) and levamisole became the main adul-
terant in cocaine seized in Switzerland (Broséus et
al., 2015b), while it was found in 48% of cocaine
hydrochloride samples tested in Spain by Energy
Control, a harm reduction organisation (Ventura
et al., 2011). By 2015, the DEA determined that
93% of 730 analysed samples from cocaine bricks
seized in the United States contained levami-
sole or PTHIT mixtures (CSP, 2016), while in the
Netherlands, about 70% of the more than 1 300
consumer cocaine hydrochloride powders tested
by the Trimbos Institute contained levamisole (Trim-
bos Instituut, 2016). It is also worth noting that
levamisole was detected in 63% of 154 cocaine
hydrochloride samples seized in Morocco between
2007 and 2016, making it by far the most frequently
found cutting agent in the study (Stambouli and
El Bouri, 2017).
In South America, however, levamisole appears
to have been detected in cocaine products, par-
ticularly consumer products, later than in North
America and Europe, although not enough data is
available to make any definite judgements. Thus,
in Chile a tiny 0.07% of 8 800 mostly consumer
cocaine hydrochloride samples analysed in 2009
contained levamisole, and a little more than 4%
of 5 500 samples in 2013 (Duffau et al., 2015). In
Brazil, none of the 513 cocaine hydrochloride sam-
ples seized in the south-eastern state of Espirito
Santo over 2008-2012 were reported to contain
levamisole, and neither were samples seized by
the Federal Police during roughly the same period,
COCAINE INSIGHTS

35
although in both cases it is unclear if the meth-
odologies used sought to identify levamisole
specifically (De Souza et al., 2016; Zacca et al.
2014).
Nevertheless, levamisole was found in seizures of
larger amounts of cocaine in Brazil, or in cocaine
destined to be shipped out of Brazil, in the late-
2000s to early 2010s. The adulterant was detected
in about 8% of 1 085 cocaine samples taken from
seizures of wholesale amounts carried out in seven
Brazilian states between 2009 and 2013 (Grobério
et al., 2015). Levamisole was also found in 19%
of 210 samples seized throughout Brazil between
2009 and 2012 (Botelho et al., 2014), and in more
than 55% of samples of cocaine departing Brazil
seized at the international airport of Sao Paulo
and in mailing services in 2011 (Lapachinske et
al., 2015). In Colombia, a little less than 8% of 65
samples from domestically produced 1-kilo cocaine
hydrochloride bricks were reported to contain
levamisole in 2009 (Garzón et al., 2009).
Interestingly, in the European countries for which
enough data is available, initial detection of levami-
sole appears to have occurred in coincidence or
close sequence with detection of hydroxyzine and
diltiazem in cocaine hydrochloride, and there are
indications that it is also the case in the United
States (McGill et al., 2008). In the Netherlands,
levamisole, diltiazem and hydroxyzine were all
detected for the first time in 2004 (Brunt et al.
2009); in Italy, levamisole and hydroxyzine were
detected in the same year (Fucci, 2007); and in
France, hydroxyzine was first detected in 2003
and diltiazem and levamisole in 2004 (Dujourdy
et al., 2010). In subsequent years, the presence
of all 3 cutting agents was detected in cocaine
hydrochloride samples in every year covered by
Dutch, French, Luxembourgish and Swiss studies
(Broséus et al., 2015b; Schneider and Meys, 2011;
Dujourdy et al, 2010; Brunt et al., 2009).
In addition, a standardized chemical analysis of
hundreds of samples of cocaine base and hydro-
chloride consumer products seized in 2012 in
Bolivia, Colombia and Peru indicates that Colombia
was the only country where levamisole, diltiazem
and hydroxyzine were found as adulterants. The
same report concluded that levamisole was the
substance most frequently used as an adulterant
in cocaine hydrochloride bricks exported to large
consumer markets (Comunidad Andina, 2013).
All this suggests that, starting around the early-
to-mid-2000s, mixtures containing diltiazem,
hydroxyzine and levamisole in different concen-
trations were added in Colombia to some cocaine
hydrochloride products destined to be exported to
Europe and North America at the request of the
owners of the cocaine and in quantities specified
by them (SIMCI, 2019b; INCB, 2018; 2017; Comu-
nidad Andina, 2013; Casale et al., 2012; Casale
et al., 2008b). Initially, such traffickers may have
been illicit actors involved in the exportation and
wholesale of cocaine products to the European and
North American markets, while buyers acting on
South American markets did not request adulter-
ants. Later on, in the early-to-mid-2010s, cocaine
adulterated with levamisole, diltiazem and hydroxy-
zine also appeared in products sold to Colombian
users (Comunidad Andina, 2013), and to users else-
where in South America (CICAD, 2019b), including
Brazil (Ribeiro de Araújo et al., 2019) and Chile
(Duffau et al., 2020).
Some recent data suggest that use of levamisole
(and other adulterants) as an additive in cocaine
products started to decrease in the mid-to-late
2010s, at least in the United States and in Europe
(no recent data is available for the rest of the world).
In 2017, for the first time since 2009, less than 50%
of the cocaine bricks seized in the United States
and analysed by the DEA contained levamisole
(CSP, 2018). The proportion of levamisole-contain-
ing bricks tested by the DEA has further declined
since, with the latest report indicating that 16% of
analysed bricks seized in the United States during
the first half of 2020 contained the substance (CSP,
2021). In Europe, the Dutch Information Monitoring
System (DIMS) reports a continuous decrease in
the proportion of thousands of consumer cocaine
hydrochloride products containing levamisole it
tested between 2015 (74%) and 2018 (35%) (Trim-
bos Instituut, 2019). The EMCDDA and Europol
(2019) also report a decrease in cocaine adultera-
tion in Europe, and under 20% of 830 cocaine
samples tested by drug checking organisations
in 7 countries in the first half of 2018 contained
levamisole (EMCDDA, 2019b).
Volume 2

36
TABLE 1 Six adulterants frequently found in cocaine powders
Adulterant Levamisole (and other PTHIT) Phenacetin Diltiazem Hydroxyzine
Lidocaine
(and similar substances)
Caffeine
Main properties
and licit uses
Anthelmintic (animals), immuno-modulator,
widely used in livestock (bovine, ovine, por-
cine), no longer used in humans due to severe
adverse effects, except in very few instances
(see box on levamisole).
Analgesic, antipyretic, no
longer used in medicine in many
countries due to adverse
effects.
Calcium channel blocker,
vasodilator, widely used in
heart medications.
Antihistamine, used as anxi-
olytic, sedative and
antiemetic.
Local anaesthetic, widely
used in dentistry and for nasal
anaesthesia.
Stimulant, widely used
in cold medicines and
energy drinks.
Possible reasons
and motivations
for presence
alongside
cocaine (other
than bulking)
Wide availability as a veterinary medicine (20),
inexpensive to buy and twice heavier than
cocaine for the same volume (19); adds a sheen
to repackaged cocaine bricks, enabling further
adulteration of cocaine products down the traf-
ficking chain (19); may potentiate or prolong the
pleasurable effects of cocaine (13, 14, 15), prob-
ably due to the action of its metabolite amino-
rex (12, 21) (see below); some users report that
they prefer cocaine hydrochloride products con-
taining levamisole to those not containing lev-
amisole (30).
Availability as an analgesic med-
icine in some South American
countries and elsewhere (6, 15);
bitter taste similar to cocaine;
enhances aspect of cocaine by
adding a shine to powders (3,
15); slight euphoric effect when
used with caffeine; may help
minimize side effects of cocaine
or another adulterant (17).
Once alleged to minimize car-
diovascular toxicity of
cocaine, but this has been
proven false; may reduce side
effects of cocaine (17); gives a
false positive for cocaine on
some colorimetric tests (16),
which may help dissimulate
the extent of cocaine adulter-
ation.
Has local anaesthetic effects
(23) (see reasons for use of
lidocaine and similar sub-
stances); long-acting sedative
effect may give users the
impression that they will be
able to sleep even after using
cocaine (17).
Disguises loss of cocaine
potency due to addition of cut-
ting agents (1); mimics numb-
ing effects of cocaine on nose
and mouth (1, 3); nasal anaes-
thetic effect may facilitate use
of large doses of cocaine (3);
gives a false positive for cocaine
on some colorimetric tests (16),
which may help dissimulate the
extent of cocaine adulteration.
Bitter taste similar to
cocaine (23, 24); similar
stimulating, yet milder,
properties as cocaine (1,
24); may enhance felt
effects of cocaine (23).
Harmful effects
associated with
prolonged use
or use in high
doses
Exact physiologic effects when used with
cocaine are not clear; levamisole affects white
blood cells; prolonged use is potentially lethal;
may enhance toxic effect of cocaine on cardio-
vascular system (17); has led to cases of severe
agranulocytosis, neutropenia, arthralgia, pulmo-
nary hypertension, retiform purpura, skin
necrosis, leukoencephalopathy (4, 7, 8, 9, 10,
18, 19, 24, 27); less severe adverse effects
include nausea, vomiting, headache, fatigue,
fever, diarrhoea, myalgia, dizziness, confusion,
and rash (24).
Toxicity on kidneys, blood and
cardiovascular system, and sus-
pected carcinogenicity (1, 3, 15);
toxicity on liver when combined
with alcohol (15).
Potentiates toxicity of
cocaine; may lead to cardio-
vascular issues including
angina, hypotension and
arrhythmia (5, 17).
Rare adverse effects include
dizziness (6) and convulsions
(15); may potentiate toxic
effects of cocaine (17).
Can potentiate toxic effects of
cocaine including cardiac
arrhythmia, convulsions and sei-
zures (3); high dose can lead to
hallucinations and seizures (22);
may cause pulmonary injury
(17). Benzocaine in large doses
can lead to methaemoglobinae-
mia, a blood condition (6).
Moderate to high doses
can lead to serious harm
including death (2); may
potentiate toxic effects of
cocaine (3); chronic use
may incur withdrawal
symptoms (6).
Notable
seizures
Bolivia reported seizing 100 kg in 2016 (28). Bolivia reported seizing about
580 kg in 2016 (28).
Brazil reported seizing
more than 2 tons in 2015
(28).
Also note
Some levamisole may be manufactured illegally
in South America (26). Levamisole metabolises
in the body into aminorex, an amphetamine-like
substance used in the past as an appetite sup-
pressant in some European countries (24); ami-
norex is suspected to have been used to dope
racing horses in North America, Western
Europe and China (27). Rare instances of illicit
manufacture of aminorex for sale as a drug
have been detected (25). Aminorex has stimu-
lating effects that are distinct and much more
potent than those of levamisole, and that are
comparable to those of cocaine (24); due to the
longer duration of its effects compared to
cocaine, it is thought to prolong the effects felt
by cocaine users (21, 24); aminorex is known to
cause hypertension but this effect has yet to be
connected to use of cocaine products (10, 21);
levamisole can be used to adulterate heroin in
Colombia (29).
One of the metabolites of phen-
acetin is acetaminophen (paracet-
amol), which is also frequently
used as an adulterant in cocaine
(and heroin) products.
Caffeine is likely to be the
substance most widely
used to adulterate illicit
drug products at global
level. In addition to
cocaine, it is also fre-
quently found in heroin,
ecstasy/MDMA, amphet-
amine and methampheta-
mine products.
COCAINE INSIGHTS

37
Sources for Table 1
1. Cole et al., 2011
2. De Sanctis et al., 2017
3. CICAD, 2019b
4. Larocque and Hoffman, 2012
5. Brunt, 2012
6. Brunt et al., 2009
7. Zhu et al., 2009
8. Knowles et al., 2009
9. Aberastury et al., 2011
10. Kachiu et al., 2012
11. Karch et al., 2011
12. Bertol et al., 2011
13.
Raymon and Isenschmid,
2009
14. Hantson, 2015
15. Comunidad Andina, 2013
16. Marcelo et al., 2016
1 7. Pawlik et al., 2015
18. Karch et al., 2014
19. Brunt et al., 2017
20. Cooper et al., 2011
21. Hofmaier et al., 2014
22. Shannon, 1988
23. Kudlachek et al., 2017
24. Solomon and Hayes, 2017
25.
Rodriguez and Allred, 2005
26. Casale et al., 2012
27. Chang et al., 2010
28. INCB, 2018
29. SIMCI, 2019b
30. EMCDDA, 2018a
Volume 2

38
References
Aberastury, M., Silva, W., Vaccarezza, M., Maxit, C. and
Agosta, G. (2011), “Epilepsia Partialis Continua Associated
with Levamisole”, Pediatric Neurology, vol. 44, issue 5,
May. Available online at: https://www.researchgate.net/
publication/51040572_Epilepsia_Partialis_Continua_Asso-
ciated_With_Levamisole (accessed 23 May 2020).
ACMD (2015), Cocaine Powder: Review of the evidence
of prevalence and patterns of use, harms, and implica-
tions, Advisory Council on the Misuse of Drugs, 12 March.
Available online at: https://assets.publishing.service.gov.
uk/government/uploads/system/uploads/attachment_data/
file/411574/acmd_final_report_12_03_2015.pdf (accessed
15 May 2020).
Adams, E. and Kozel, N. (1985), “Cocaine Use in America:
Introduction and Overview”, in Kozel, N. and Adams, E.
(eds.), Cocaine Use in America: Epidemiologic and Clini-
cal Perspectives, NIDA Research monograph, National
Institute on Drug Abuse, Washington, D.C.
Andreasen, M. Lindholst, C. and Kaa, E. (2009), “Adulter-
ants and Diluents in Heroin, Amphetamine, and Cocaine
Found on the Illicit Drug Market in Aarhus, Denmark”, The
Open Forensic Science Journal, vol. 2.
ANSM (2014), Compte rendu de séance, Comité technique
des Centres d’évaluation et d’information sur la phar-
macodépendance – CT022014023, Agence nationale de
sécurité du médicament et des produits de santé, 3 April.
Available online at: https://ansm.sante.fr/var/ansm_site/
storage/original/application/8686ea4eb4638fbbe5caade
3c53f0f9e.pdf (accessed 10 April 2020).
Anonymous (1982), “Part IV: Cocaine Free Base Abuse: A
New Smoking Disorder”, Journal of Psychoactive Drugs,
vol. 14, issue 4.
Anvil, J. (1979), Everything You’ll ever Need to Know about
Freebase Cocaine, the Greatest Thing since Sex, World’s Fair
Publishing Company. Available online at: https://archive.org/
stream/FreeBase_282/1-28_djvu.txt (accessed 27 April 2020).
Arias, A., García Godoy, B. and Manes, R. (coord.) (2014),
Trabajos seleccionados: V Encuentro Internacional de
Políticas Públicas y Trabajo Social: debates en torno a
la construcción de institucionalidad, Departamento de
Publicaciones de la Facultad de Derecho y Ciencias Soci-
ales de la Universidad de Buenos Aires, Buenos Aires.
Available online at: http://intercambios.org.ar/publicacio-
nes/2014%20Pawlowicz%20Madres%20del%20Paco%20
libro%20completo.pdf (accessed 13 May 2020).
Barbera, N., Busardò, F., Indorato, F. and Romano, G.
(2013), “The pathogenetic role of adulterants in 5 cases
of drug addicts with a fatal outcome”. Forensic Science
International, vol. 227, issues 1-3, April.
Barrio, G., Saavedra, P., de la Fuente, L. and Royuela, L.
(1997), “Purity of cocaine seized in Spain, 1985–1993: varia-
tions by weight, province and year of seizure”, Forensic
Science International, vol. 85, issue 1, February.
Bastos, F., Mendes, A., Arruda Vieira Duarte, P. and Ber-
toni, N. (2011), “Smoked crack cocaine in contemporary
Brazil: the emergence and spread of ‘oxi’”, Addiction, 106.
Available online at: https://onlinelibrary.wiley.com/doi/
epdf/10.1111/j.1360-0443.2011.03427.x (accessed 3 April
2020).
Bastos, F. and Bertoni, N. (org.) (2014), Pesquisa Nacional
sobre o uso de crack: quem são os usuários de crack e/
ou similares do Brasil? Quantos são nas capitais brasilei-
ras?, ICICT/FIOCRUZ, Rio de Janeiro. Available online at :
https://www.icict.fiocruz.br/sites/www.icict.fiocruz.br/files/
Pesquisa%20Nacional%20sobre%20o%20Uso%20de%20
Crack.pdf (accessed 12 April 2020).
BBC (n.d.), Full list of impurities found in cocaine, web-
page, British Broadcasting Corporation. Available online
at: http://news.bbc.co.uk/1/hi/uk/8040690.stm (accessed
17 May 2020).
Bean, P., “Cocaine and Crack: An Introduction”, in Bean,
P. (ed.) (1993), Cocaine and Crack: Supply and Use, St.
Martin’s Press, New York.
Behrman, A. (2008), “Luck of the Draw: Common Adulter-
ants Found in Illicit Drugs”, Journal of Emergency Nursing,
vol. 34, issue 1, February.
Benowitz, N. (1993), “Clinical pharmacology and toxicol-
ogy of cocaine”, Pharmacology & Toxicology, vol. 72, issue
1, January.
Bernardo, N., Siqueira, M., de Paiva, M. and Maia, P.
(2003), “Caffeine and other adulterants in seizures of street
cocaine in Brazil”, International Journal of Drug Policy, vol.
14, issue 4, August.
Bertol, E., Mari, M., DiMilia, L., Politi, S., Furlanetto, S. and
Karch, S. (2011), “Determination of aminorex in human
urine samples by GC-MS after use of levamisole”, Jour-
nal of Pharmaceutical and Biomedical Analysis, vol. 55,
issue 5, July.
Bertol, E., Bigagli, L., D’Errico, S., Mari, F., Palumbo, D.,
Pascali, J. P. and Vaiano, F. (2018), Analysis of illicit drugs
seized in the Province of Florence from 2006 to 2016”,
Forensic Science International, vol. 284, March.
COCAINE INSIGHTS

39
BFP (2021a), “Cocaine Production Sites in Brazil - Chemi-
cal Profiling Tools”, Serviço de Pericias de Laboratório e de
Balística (PeQui Project), Brazilian Federal Police. Presen-
tation at the UNODC Technical Meeting on Coca/Cocaine
Production Sites, 17 June.
BFP (2021b), Serviço de Pericias de Laboratório e de
Balística (PeQui Project), Brazilian Federal Police. Personal
communication, 20 July.
Blickman, T. (20 06), Smokeable cocaine and crack in Brazil.
A quick scan of the market, Research Paper, Transnational
Institute (TNI), October. Available online at: https://www.
tni.org/files/crack-brazil.pdf (accessed 25 april 2020).
Bono, J. (2008), “The Criminalistics of Controlled Sub-
stances”, in Karch, S. (2008) (ed.), Pathology, Toxicogenetics,
and Criminalistics of Drug Abuse, CRC Press, Boca Raton.
Botelho, E., Cunha, R., Campos, A. and Maldaner, A. (2014),
“Chemical Profiling of Cocaine Seized by Brazilian Fed-
eral Police in 2009-2012: Major Components”, Journal of
the Brazilian Chemical Society, vol. 25, n° 4. Available
online at: http://static.sites.sbq.org.br/jbcs.sbq.org.br/pdf/
v25n4a01.pdf (accessed 5 May 2020).
Broséus, J., Huhtala, S. and Esseiva, P. (2015a), “First sys-
tematic chemical profiling of cocaine police seizures in
Finland in the framework of an intelligence-led approach”,
Forensic Science International, vol. 251, June.
Broséus, J., Gentile, N., Bonadio Pont, F., Garcia Gongora,
J., Gasté, L. and Esseiva, P. (2015b), “Qualitative, quanti-
tative and temporal study of cutting agents for cocaine
and heroin over 9 years”, Forensic Science International,
vol. 257, December.
Broséus, J., Gentile, N. and Esseiva, P. (2016), “The cutting
of cocaine and heroin: A critical review”, Forensic Science
International, vol. 262, May.
Brunt, T. (2012), Monitoring illicit psychostimulants and
related health issues, Ph.D. Dissertation, University of
Amsterdam, Amsterdam, March. Available online at:
https://pure.uva.nl/ws/files/1611812/103269_thesis.pdf
(accessed 7 May 2020).
Brunt, T. (2017), Drug checking as a harm reduction tool
for recreational drug users: opportunities and challenges,
Background paper commissioned by the EMCDDA for
Health and social responses to drug problems: a Euro-
pean guide, 30 October. Available online at: https://
www.emcdda.europa.eu/system/files/attachments/6339/
EuropeanResponsesGuide2017_BackgroundPaper-Drug-
checking-harm-reduction_0.pdf (accessed 10 May 2020).
Brunt, T., Rigter, S., Hoek, J., Vogels, N., van Dijk, P. and
Niesink, R. (2009), “An analysis of cocaine powder in the
Netherlands: content and health hazards due to adulter-
ants”, Addiction, vol. 104, n° 5.
Brunt, T., Nagy, C., Bücheli, A., Martins, D., Ugarte, M.,
Beduwe, C. and Ventura, M. (2016), “Drug testing in
Europe: monitoring results of the Trans European Drug
Information (TEDI) project”, Drug Testing and Analysis,
vol. 9 issue 2, February.
Brunt, T., van den Berg, J., Pennings, E. and Venhuis, B.
(2017), “Adverse effects of levamisole in cocaine users: a
review and risk assessment”, Archives of Toxicology, vol.
91, issue 6, June.
Buchanan, J., Oyer, R., Patel, N., Jacquet, G., Bornikova,
L., Thienelt, C., Shriver, D., Shockley, L., Wilson, M.,
Hurlbut, K. and Lavonas, E. (2010), “A Confirmed Case
of Agranulocytosis after Use of Cocaine Contaminated
with Levamisole”, Journal of Medical Toxicology, vol. 6,
issue 2, June. Available online at: https://link.springer.com/
content/pdf/10.1007%2Fs13181-010-0060-3.pdf (accessed
8 May 2020).
Busardò, F., Pichini, S., Pacifici, R. and Karch, S. (2016),
“The Never-Ending Public Health Issue of Adulterants in
Abused Drugs”, Journal of Analytical Toxicology, vol. 40,
issue 7, September.
By, A., Lodge, B. and Sy, W. (1988), “Characterization of
Cis-Cinnamoylcocaine”, Canadian Society of Forensic Sci-
ence Journal, vol. 21, n° 1-2.
Campos Neto, M., Vanrell, J. and Calvo, C. (2012),
“Modalidades no Transporte da Pasta Base (Cocaína)
nas Fronteiras do Oeste de Mato Grosso (Brasil-Bolívia)”,
Brazilian Journal of Forensic Sciences, Medical Law and
Bioethics, vol. 1, n° 3.
Capece, J. (2008), “Repensando las adicciones: el para
-
digma cognitivo y el trastorno por dependencia”, Vertex,
vol. XIX, n° 77, January-March.
Casale, J. and Klein, R. (1993), “Illicit production of
cocaine”, Forensic Science Review, vol. 5, n° 2.
Casale, J., Hays, P., Toske, S. and Berrier, A. (2007), “Four
New Illicit Cocaine Impurities from the Oxidation of Crude
Cocaine Base: Formation and Characterization of the Dia-
stereomeric 2,3-Dihydroxy-3-Phenylpropionylecgonine
Methyl Esters from cis- and trans-Cinnamoylcocaine”,
Journal of Forensic Science, vol. 52, n° 4.
Casale, J., Boudreau, D. and Jones, L. (2008a), “Tropane
Ethyl Esters in Illicit Cocaine: Isolation, Detection, and
Determination of New Manufacturing By-Products from
the Clandestine Purification of Crude Cocaine Base with
Ethanol”, Journal of Forensic Science, vol. 53, n° 3, May.
Volume 2

40
Casale, J., Corbeil, E. and Hays, P. (2008b), “Identification
of Levamisole Impurities Found in Illicit Cocaine Exhibits”,
Microgram Journal, vol. 6, n° 3-4, July-December.
Casale, J., Colley, V. and LeGatt, D. (2012), “Determina-
tion of Phenyltetrahydroimidazothiazole Enantiomers
(Levamisole/Dexamisole) in Illicit Cocaine Seizures and
in the Urine of Cocaine Abusers via Chiral Capillary Gas
Chromatography–Flame-Ionization Detection: Clinical and
Forensic Perspectives”, Journal of Analytical Toxicology,
vol. 36, n°2, March.
Casale, J., Mallette, J. and Jones, L. (2014), “Chemo-
systematic identification of fifteen new cocaine-bearing
Erythroxylum cultigens grown in Colombia for illicit
cocaine production”, Forensic Science International, vol.
237, April.
Castaño, G. (2000), “Cocaínas fumables en Latinoamérica”,
Adicciones, vol. 12, n° 4. Available online at : http://www.
adicciones.es/index.php/adicciones/article/view/664/653
(accessed 12 April 2020).
Caudevilla, F., Ventura, M., Fornís, I., Barratt, M. J., Vidal,
C., Lladanosa, C., and Calzada, N. (2016), “Results of an
international drug testing service for cryptomarket users”,
International Journal of Drug Policy, vol. 35, September.
Caulkins, J. and Reuter, P. (1998), “What Price Data Tell
Us about Drug Markets”, Journal of Drug Issues, vol. 28,
issue 3, July.
Chang, A., Osterloh, J. and Thomas, J. (2010), “Levami-
sole: A Dangerous New Cocaine Adulterant”, Clinical
Pharmacology & Therapeutics, vol. 88, n° 3.
Chiarotti, M., Marsili, R. and Moreda-Piñeiro, A. (2002),
“Gas chromatographic–mass spectrometric analysis of
residual solvent trapped into illicit cocaine exhibits using
head-space solid-phase microextraction”, Journal of Chro-
matography B, vol. 772, issue 2, June.
CICAD (2003), Estudio Comparativo del Consumo de
Drogas en Países Americanos, Organisation of American
States, Inter-American Drug Abuse Control Commission,
Washington, D.C.
CICAD (2014), Cocaine base paste consumption in South
America: a review of epidemiological and medical-tox-
icological aspects, Organisation of American States,
Inter-American Drug Abuse Control Commission, Wash-
ington, D.C.
CICAD (2016a), Subregional compendium: Analysis of the
chemical composition of smokable cocaine substances,
Organisation of American States, Inter-American Drug
Abuse Control Commission, Inter-American Observatory
on Drugs, Washington, D.C., May.
CICAD (2016b), Compendio subregional: Análisis de car-
acterización química de cocaínas fumables:, Organisation
of American States, Inter-American Drug Abuse Control
Commission, Inter-American Observatory on Drugs, Wash-
ington, D.C., May.
CICAD (2019a), Informe sobre el consumo de drogas en las
Américas, 2019, Organización de los Estados Americanos,
Comisión Interamericana para el Control del Abuso de
Drogas, Washington, D.C.
CICAD (2019b), Drug adulterants and their effects on the
health of users: a critical review, Organización de los Esta-
dos Americanos, Comisión Interamericana para el Control
del Abuso de Drogas, Washington, D.C. Available online
at: http://www.cicad.oas.org/oid/pubs/Final%20ENG%20
Drug%20adulterants%20and%20their%20effects%20
on%20the%20health%20of%20users%20-%20a%20.._.pdf
(accessed 20 March 2020).
Cole, C., Jones, L., McVeigh, J., Kicman, A., Syed, Q. and
Bellis, M. (2010), CUT: A Guide to Adulterants, Bulking
Agents and Other Contaminants Found in Illicit Drugs,
Faculty of Health and Applied Social Sciences, Liverpool
John Moores University, Liverpool. Available online at:
https://www.drugsandalcohol.ie/13119/1/Cut_a_guide_to_
adulterants.pdf (accessed 7 May 2020).
Cole, C., Jones, L., McVeigh, J., Kicman, A., Syed, Q. and
Bellis, M. (2011), “Adulterants in illicit drugs: a review of
empirical evidence”, Drug Testing and Analysis, vol. 3 issue
2, February.
Colley, V. and Casale, J. (2014), “Differentiation of South
American crack and domestic (US) crack cocaine via
headspace-gas chromatography/mass spectrometry”, Drug
Testing and Analysis, vol. 7, issue 3, October.
Colussi-Mas, J., Bellemin, B., Bernard, N. and Descotes,
J. (2003), “Le crack : une forme fumable de cocaïne”, La
Lettre du Pharmacologue, vol. 17, n° 5, Oct-Nov-Dec.
Comunidad Andina (2013), Caracterización química
de drogas cocaínicas, incautadas, en 27 ciudades de
la subregión andina, Bolivia, Colombia y Perú 2012.
Informe Regional, Secretaría General de la Comuni-
dad Andina, Unión Europea, Lima, February. Available
online at: http://www.comunidadandina.org/DS/Inf.%20
Caracterización%20Regional.pdf (accessed 2 May
2020).
CONACE (2004), Sexto Estudio Nacional de Drogas
en Población General de Chile, Consejo Nacional
para el Control de Estupefacientes, Ministerio del
Interior, Gobierno de Chile, Santiago de Chile. Avail-
able online at: https://www.senda.gob.cl/wp-content/
uploads/2019/07/VI-ESTUDIO-GENERAL-2004.pdf
(accessed 4 May 2020).
COCAINE INSIGHTS

41
Cone, E. (1995), “Pharmacokinetics and Pharmacodynam-
ics of Cocaine”, Journal of Analytical Toxicology, vol. 19,
issue 6, October.
Cooper, K., Whelan, M., Danaher, M. and Kennedy, D.
(2011), “Stability during cooking of anthelmintic veterinary
drug residues in beef”, Food Additives & Contaminants:
Part A, vol. 28, n° 2, February.
Cortés, E. (n.d.), Comprando Miedo: Personas usuarias
de crack en Costa Rica, position paper, Asociación Costar-
ricense para el Estudio e Intervención en Drogas (ACEID),
Red Latinoamericana y del Caribe de Personas que Usan
Drogas (LANPUD). Available online at: http://fileserver.
idpc.net/library/Personas_usuarias_de_crack_en_Costa_
Rica.pdf (accessed 6 June 2020).
CSP (2016), Cocaine Signature Program Report, Drug
Enforcement Administration, Special Testing and Research
Laboratory, July.
CSP (2018), Cocaine Signature Program Report, Drug
Enforcement Administration, Special Testing and Research
Laboratory, January.
CSP (2020), Cocaine Signature Program Report, Drug
Enforcement Administration, Special Testing and Research
Laboratory, January.
CSP (2021), Cocaine Signature Program Report, Drug
Enforcement Administration, Special Testing and Research
Laboratory, January.
Cui, X., Wang, R., Lian, R., Liang, C., Chen, G. and Zhang,
Y. (2019), “Correlation analysis between cocaine samples
seized in China by the rapid detection of organic impurities
using direct analysis in real time coupled with high-reso-
lution mass spectrometry”, International Journal of Mass
Spectrometry, vol. 444, October.
Cunningham, E., Venuto, R. and Zielezny, M. (1984),
“Adulterants in heroin/cocaine: Implications concerning
heroin-associated nephropathy”, Drug and Alcohol Depen-
dence, vol. 14, issue 1, September.
Cunningham, J., Maxwell, J., Campollo, O., Cunningham,
K., Liu, L.-M. and Lin, H.-L. (2010), “Proximity to the US-
Mexico border: a key to explaining geographic variation in
US methamphetamine, cocaine and heroin purity”, Addic-
tion, vol. 105, n° 10.
Curini, R., Zamponi, S., D’ascenzo, F., De Angelis Curtis,
S., Marino, A. and Dezzi, A. (1989), “Thermal analytical
techniques applied to the narcotic field: cocaine analysis”.
Thermochimica Acta, vol. 153, November.
Da Silva Júnior, R., Gomes, C., Goulart Júnior, S., Almeida,
F., Grobério, T., Braga, J., Zacca, J., Vieira, M., Botelho,
E. and Maldaner, A. (2012), “Demystifying ‘oxi’ cocaine:
Chemical profiling analysis of a ‘new Brazilian drug’ from
Acre State”, Forensic Science International, vol. 221, issues
1-3, September.
Da Silva, A., Grobério, T., Zacca, J, Maldaner, A. and Braga,
J. (2018), “Cocaine and adulterants analysis in seized drug
samples by infrared spectroscopy and MCR-ALS”, Forensic
Science International, vol. 290, September.
Dávila, L., Solórzano, E., Premoli de Percoco, G., Quiño-
nes, B. and Petrosino, P. (2001), “El consumo de basuco
como agente causal de alteraciones en la encía”, Revista
Cubana de Estomatología, vol. 38, n° 2. Available online at:
http://www.revestomatologia.sld.cu/index.php/est/article/
view/2323/554 (accessed 22 July 2020).
DEA (2015), 2013 National Level STRIDE Price and Purity
Data, Drug Enforcement Administration, Unclassified,
September. Available online at: https://ndews.umd.edu/
sites/ndews.umd.edu/files/pubs/2013%20National%20
Level%20STRIDE%20Price%20and%20Purity%20Data.pdf
(accessed 27April 2020).
DEA (2019), National Drug Threat Assessment 2019, Drug
Enforcement Administration, U.S. Department of Justice,
Washington, D.C., December. Available online at: https://
www.dea.gov/sites/default/files/2020-01/2019-NDTA-
final-01-14-2020_Low_Web-DIR-007-20_2019.pdf (accessed
24 March 2020).
Decorte, T. (2001), “Quality Control by Cocaine Users:
Underdeveloped Harm Reduction Strategies”, European
Addiction Research, vol. 7, n° 4. Available online at: https://
www.jstor.org/stable/26790164?seq=1#metadata_info_
tab_contents (accessed 14 May 2020).
De Sanctis V., Soliman N., Soliman A., Elsedfy, H., Di
Maio, S., El Kholy, M. and Fiscina, B. (2017), “Caffein-
ated energy drink consumption among adolescents and
potential health consequences associated with their use:
a significant public health hazard”, Acta Biomedica, vol.
88, issue 2, August.
De Souza, L. (2014), Fingerprinting de Cocaína: Um
Estudo do Perfil Químico no Estado do Espírito Santo,
Dissertação de Mestrado em Química, Universidade
Federal do Espírito Santo, Centro de Ciências Exatas,
Vitória. Available online at: http://repositorio.ufes.br/bit-
stream/10/1839/1/tese_7529_Lindamara%20Maria%20
de%20Souza20140630-82317.pdf (accessed 20 April 2020).
De Souza, L., Rodrigues, R., Santos, H., Costa, H., Merlo,
B., Filgueiras, P., Poppi, R., Vaz, B. and Romão, W. (2016),
“A survey of adulterants used to cut cocaine in samples
seized in the Espírito Santo State by GC–MS allied to
chemometric tools”, Science & Justice, vol. 56, issue
2, March.
Volume 2

42
Dormitzer, C., González, G., Penna, M., Bejarano, J.,
Obando, P., Sánchez, M., Vittetoe, K., Gutiérrez, U., Al-faro,
J., Meneses, G., Bolívar Díaz, J., Herrera, M., Hasbun, J.,
Chisman, A., Caris, L., Chen, C. and Anthony, J. (2004), “The
PACARDO research project: youthful drug involvement in
Central America and the Dominican Republic”, Revista
Panameña de Salud Pública, vol. 15, n° 6. Available online
at: https://www.researchgate.net/publication/8436697_
The_PACARDO_research_project_Youthful_drug_involve-
ment_in_Central_America_and_the_Dominican_Republic
(accessed 15 May 2020).
DrugsData (n.d.), About Tests and Data, online page,
DrugsData.org (Erowid’s anonymous drug analysis pro-
gram). Available online at: https://www.ecstasydata.org/
about_data.php (accessed 15 May 2020).
Duffau, B., Rojas, S., Espinoza, M., Jofré, S. and Muñoz,
L. (2014), “Estudio de la composición química de incau-
taciones de cocaína en Chile mediante HPTLC, GC/FID
y FTIR”, Revista de Toxicología en Línea (RETEL), vol.
42. Available online at : https://www.researchgate.net/
publication/263721749_Estudio_de_la_composicion_qui-
mica_de_incautaciones_de_cocaina_en_Chile_mediante_
HPTLC_GCFID_y_FTIR (accessed 17 May 2020).
Duffau, B., Rojas, S., Fuentes, P. and Triviño, I. (2015),
“Perfil de composición de la cocaína de diseño en Chile:
estado y los peligros asociados a la adulteración con
levamisol”, Revista Chilena de Salud Pública, vol . 19 n°
1. Available online at: https://revistasaludpublica.uchile.
cl/index.php/RCSP/article/view/36350/37995 (accessed
12 May 2020).
Duffau, B., Rojas, S. and Ayala, S. (2020), “A decade
of analysis of illicit street cocaine in Chile”, Journal of
Pharmacy & Pharmacognosy Research, vol. 8, issue 2,
March-April. Available online at: http://jppres.com/jppres/
pdf/vol8/jppres19.638_8.2.146.pdf (accessed 12 May 2020).
Dujourdy, L. and Besacier, F. (2008), “Headspace profiling
of cocaine samples for intelligence purposes”, Forensic
Science International, vol. 179, issues 2-3, 6 August.
Dujourdy, L. and Besacier, F. (2010), “L’héroïne saisie en
France. Données statistiques issues de la base nationale
des laboratoires de police scientifique”, Annales Pharma-
ceutiques Françaises, vol. 68, n° 2, March.
Dujourdy, L., Besacier, F. and Ladroue, V. (2010), “La
cocaïne saisie en France. Exploitation des données
statistiques nationales”, L’actualité chimique, n° 342-343,
June-July-August.
Eiden, C., Diot, C., Mathieu, O., Mallaret, M. and Peyrière,
H. (2014), “Levamisole-Adulterated Cocaine: What about
in European Countries?” Journal of Psychoactive Drugs,
vol. 46, issue 5, November.
ElSohly, M., Brenneisen, R. and Jones, A. (1991), “Coca
Paste: Chemical Analysis and Smoking Experiments”, Jour-
nal of Forensic Sciences, vol. 36, n° 1, January.
EMCDDA (2001), Cocaine and ‘base/crack’ cocaine,
EMCDDA 2001 selected issue, EMCDDA 2001 Annual report
on the state of the drugs problem in the European Union,
Lisbon. Available online at: https://www.emcdda.europa.
eu/system/files/publications/198/sel2001_1en_69449.pdf
(accessed 2nd July 2020).
EMCDDA (2018a), Recent changes in Europe’s cocaine
market: results from an EMCDDA trendspotter study,
Publications Office of the European Union, Luxembourg.
Available online at: http://www.emcdda.europa.eu/system/
files/publications/10225/2018-cocaine-trendspotter-rapid-
communication.pdf (accessed 24 March 2020).
EMCDDA (2018b), European Drug Report 2018: Trends and
Developments, Publications Office of the European Union,
Luxembourg. Available online at: https://www.emcdda.
europa.eu/system/files/publications/8585/20181816_
TDAT18001ENN_PDF.pdf (accessed 15 May 2020).
EMCDDA (2019a), Drugs in syringes from six European
cities: results from the ESCAPE project 2017, Publications
Office of the European Union, Luxembourg. Available
online at: http://www.emcdda.europa.eu/system/files/
publications/11287/20191061_TD0119176ENN_PDF.pdf
(accessed 25 March 2020).
EMCDDA (2019b), European Drug Report 2019: Trends and
Developments, Publications Office of the European Union,
Luxembourg. Available online at: http://www.emcdda.
europa.eu/system/files/publications/11364/20191724_
TDAT19001ENN_PDF.pdf (accessed 19 April 2020).
EMCDDA (2021), Statistical Bulletin 2021 - Treatment
Demand, available online at: https://www.emcdda.europa.
eu/data/stats2021/tdi_en (accessed 22 July 2021).
EMCDDA and Europol (2016), EU Drug Markets Report:
In-Depth Analysis, European Monitoring Centre for Drugs
and Drug Addiction and Europol, Publications Office of the
European Union, Luxembourg. Available online at: http://
www.emcdda.europa.eu/system/files/publications/2373/
TD0216072ENN.PDF (accessed 12 May 2020).
EMCDDA and Europol (2019), EU Drug Markets Report 2019,
European Monitoring Centre for Drugs and Drug Addiction
and Europol, Publications Office of the European Union,
Luxembourg. Available online at: http://www.emcdda.
europa.eu/system/files/publications/12078/20192630_
TD0319332ENN_PDF.pdf (accessed 15 April 2020).
Evrard, I., Legleye, S. and Cadet-Taïrou, A. (2010), “Com-
position, purity and perceived quality of street cocaine in
France”, International Journal of Drug Policy, vol. 21, issue 5.
COCAINE INSIGHTS

43
Farrar, H. and Learns, G. (1989), “Cocaine’ Clinical phar-
macology and toxicology”, The Journal of Pediatrics, vol.
115, n° 5, Part 1, November.
Fattinger, K., Benowitz, N. Jones, R. and Verotta, D. (2000),
“Nasal mucosal versus gastrointestinal absorption of
nasally administered cocaine”, European Journal of Clini-
cal Pharmacology, vol. 56, issue 4, July.
Fiorentin, T., Fogarty, M., Limberger, R. and Logan, B.
(2019), “Determination of Cutting Agents in Seized Cocaine
Samples Using GC-MS, GC-TMS AND LC-MS/MS”, Foren-
sic Science International, vol. 295, February.
Fischer, B., Kuganesan, S., Burnett, C., Gallassi, A. and
Werb, D. (2016), ‘Crack’: Global Epidemiology, Key
Characteristics and Consequences of Use, and Existent
Interventions – a review, Beckley Foundation. Available
online at: https://beckleyfoundation.org/wp-content/
uploads/2016/04/4.-Crack-Fischer-web.pdf (accessed 29
April 2020).
Floriani, G., Gasparetto, J., Pontarolo, R. and Gonçalves,
A. (2014), “Development and validation of an HPLC-DAD
method for simultaneous determination of cocaine, ben-
zoic acid, benzoylecgonine and the main adulterants found
in products based on cocaine”, Forensic Science Interna-
tional, vol. 235, February.
Freye E. (2009), Pharmacology and Abuse of Cocaine,
Amphetamines, Ecstasy and Related Designer Drugs,
Springer, Dordrecht.
Fucci, N. and De Giovanni, N. (1998), “Adulterants encoun-
tered in the illicit cocaine market”, Forensic Science
International, vol. 95, issue 3, August.
Fucci, N. (2004), “Phenacetin and cocaine in a body packer”,
Forensic Science International, vol. 141, issue 1, April.
Fucci, N. (2007), “Unusual adulterants in cocaine seizured
on Italian clandestine market”, Forensic Science Interna-
tional, vol. 172, issues 2-3, October.
Fukushima, A., Carvalho, V., Carvalho, D., Diaz, E., Vega
Bustillos, J., Spinosa, H. and Chasine, A. (2014), “Purity
and adulterant analysis of crack seizures in Brazil”, Forensic
Science International, vol. 243, October.
Garzón, W., Parada, F. and Florián, N. (2009), “Análisis
forense de muestras de cocaína producidas en Colom-
bia: I. Perfil cromatográfico de muestras de clorhidrato
de cocaína”, Vitae, vol. 16, n° 2, July. Available online
at : https://revistas.udea.edu.co/index.php/vitae/article/
view/1934/1594 (accessed 13 May 2020).
Gold, M., Washton, A. and Dackis, C. (1985), “Cocaine
Abuse: Neurochemistry, Phenomenology, and Treatment”,
in Kozel, N. and Adams, E. (eds.), Cocaine Use in America:
Epidemiologic and Clinical Perspectives, NIDA Research
monograph, National Institute on Drug Abuse, Washing-
ton, D.C.
Goldstein, P., Brownstein, H., Ryan, P. and Bellucci, P.
(1988), “Crack and Homicide in New York City, 1988: A
Conceptually Based Event Analysis”
Gómez, J. and Rodríguez, A. (1989), “An evaluation of
the results of a drug sample analysis”, Bulletin on Nar-
cotics, vol. 41, issue 1. Available online at: https://www.
unodc.org/unodc/en/data-and-analysis/bulletin/bulle-
tin_1989-01-01_1_page013.html (accessed 1 May 2020).
Gonçalves de Carvalho, D. and Mídio, F. (2003), “Quality
of cocaine seized in 1997 in the street-drug market of São
Paulo city, Brazil”, Brazilian Journal of Pharmaceutical
Sciences, vol. 39, n° 1, January/March.
Gooteberg, P. (2008), Andean cocaine: the making of a global
drug, The University of North Carolina Press, Chapel Hill.
Gostic, T., Klemenc, S.and Štefane, B. (2009), “A study of
the thermal decomposition of adulterated cocaine samples
under optimized aerobic pyrolytic conditions”, Forensic
Science International, vol. 187, issues 1-3, May.
Gottlieb, A. (1976), The Pleasures of Cocaine, Twenty-First
Century Alchemist, Manhattan Beach, California.
Grobério, T., Zacca, J., Botelho, E., Talhavini, M. and Braga,
J. (2015), “Discrimination and quantification of cocaine
and adulterants in seized drug samples by infrared spec-
troscopy and PLSR”, Forensic Science International, vol.
257, December.
Hammond, B. and Craven, J. (2017), “Levamisole-Adulter-
ated Cocaine Leading to Fatal Vasculitis: A Case Report”,
Critical Care Nurse, vol. 37, issue 4, August.
Hantson, P. (2015), “Adultération de la cocaïne par le
lévamisole : quels risques ?” Toxicologie Analytique et
Clinique, vol. 27, issue 4, December.
Hatsukami D. and Fischman, M. (1996) “Crack Cocaine
and Cocaine Hydrochloride: Are the Differences Myth or
Reality?” Journal of the American Medical Association,
vol. 276, n° 19.
Henman, A. (2015), Report on smokable forms of cocaine in
Lima, Peru, Report for the Transnational Institute, Amster-
dam. Available online at: http://www.mamacoca.org/
docs_de_base/Consumo/anthony_henman__report_for_
tni_smokable_cocoaine_2015.html (accessed 13 July 2020).
Hofmaier, T., Luf, A., Seddik, A., Stockner, T., Holy, M., Fre-
issmuth, M., Ecker, G., Schmid, R., Sitte, H. and Kudlacek,
Volume 2

44
O. (2014), “Aminorex, a metabolite of the cocaine adul-
terant levamisole, exerts amphetamine like actions at
monoamine transporters”, Neurochemistry International,
vol. 73, July. Available online at: https://www.science-
direct.com/journal/neurochemistry-international/vol/73/
suppl/C (accessed 25 May 2020).
IHME (2021), Global Burden of Disease Study 2019 Data
Resources: GBD Results Tools. Institute for Health Metrics
and Evaluation. Available online at: http://ghdx.healthdata.
org/gbd-results-tool (accessed 23 July 2021).
INCB (2010), Precursors and chemicals frequently used in
the illicit manufacture of narcotic drugs and psychotropic
substances 2009, International Narcotics Control Board,
Vienna, 24 February.
INCB (2017), Precursors and chemicals frequently used in
the illicit manufacture of narcotic drugs and psychotropic
substances 2016, International Narcotics Control Board,
Vienna, 2nd March.
INCB (2018), Precursors and chemicals frequently used in
the illicit manufacture of narcotic drugs and psychotropic
substances 2017, International Narcotics Control Board,
Vienna, 1st March.
INCB (2019), Precursors and chemicals frequently used in
the illicit manufacture of narcotic drugs and psychotropic
substances 2018, International Narcotics Control Board,
Vienna, 5 March.
INCB (2020), Precursors and chemicals frequently used in
the illicit manufacture of narcotic drugs and psychotropic
substances 2019, International Narcotics Control Board,
Vienna, 27 February.
INCHEM (1993), Cocaïne (PIM 139F), Internationally Peer-
Reviewed Chemical Safety Information, International
Programme on Chemical Safety. Available online at: http://
www.inchem.org/documents/pims/pharm/pim139f.htm
(accessed 6 April 2020).
Indorato, F., Romano, G. and Barbera, N. (2016), “Levam-
isole-adulterated cocaine: Two fatal case reports and
evaluation of possible cocaine toxicity potentiation”, Foren-
sic Science International, vol. 265, August.
Janssen, E., Cadet-Taïrou, A., Gérome, C. and Vuolo,
M. (2020), “Estimating the size of crack cocaine users
in France: Methods for an elusive population with high
heterogeneity”,
International Journal of Drug Policy, vol. 76, February.
Janzen, K. (2013), “Cross-Matching of Cocaine Samples.
A Case Study”, Canadian Society of Forensic Science Jour-
nal, vol. 20, n° 2.
Jekel, J., Allen, D., Clarke, N, Podlewski, H., Gray, H. and
Roberts, C. (1994), “Nine Years of the Freebase Cocaine
Epidemic in the Bahamas”, The American Journal on
Addictions, vol. 3, n° 1, Winter.
Jeri, F. (1984), “Coca-paste smoking in some Latin Ameri-
can countries: a severe and unabated form of addiction”,
Bulletin on Narcotics, Issue 2. Available online at: https://
www.unodc.org/unodc/en/data-and-analysis/bulletin/bul
-
letin_1984-01-01_2_page003.html (accessed 14 April 2020).
Jeri, F., Sánchez, C., del Pozo, C., Fernández, M. and Car-
bajal, C. (1978), “Further experience with the syndromes
produced by coca paste smoking”, Bulletin on Narcotics,
Issue 3. Available online at: https://www.unodc.org/unodc/
en/data-and-analysis/bulletin/bulletin_1978-01-01_3_
page002.html (accessed 14 April 2020).
JND (2006), Pasta Base de Cocaína – Prácticas y Gestión
de riesgos en adolescentes uruguayos, Junta Nacional
de Drogas, Montevideo. Available online at : https://www.
researchgate.net/publication/299537624_Pasta_Base_de_
Cocaina-_Practicas_y_Gestion_de_riesgos_en_adolescen-
tes_uruguayos (accessed 23 July 2020).
JND (2013), Estudios de seroprevalencia de vih/sida
y de conocimientos, actitudes y prácticas entre usu-
arios de pasta base, crack y otras denominaciones de
la cocaína fumable en Montevideo y su área metropoli-
tana, Junta Nacional de Drogas, Montevideo. Available
online at : https://www.gub.uy/junta-nacional-drogas/sites/
junta-nacional-drogas/files/2018-01/estudio_vih_sida_
cocaina_fumable.pdf (accessed 11 July 2020).
JND (2019), Personas, calle, consumos: dos estudios
sobre uso de pasta base en Uruguay, Junta Nacional de
Drogas, Montevideo. Available online at : https://www.gub.
uy/junta-nacional-drogas/comunicacion/noticias/nuevo-
estudio-sobre-pasta-base-cocaina (accessed 11 July 2020).
Johnson, M., Hermann, E., Sweeney, M., LeComte, R. and
Johnson, P. (2016), “Cocaine administration dose-depend-
ently increases sexual desire and decreases condom use
likelihood: The role of delay and probability discounting
in connecting cocaine with HIV”, Psychopharmacology,
vol. 234, issue 4, December.
Kachiu, C., Ladizinski, B. and Federman, D. (2012), “Compli-
cations Associated with Use of Levamisole-Contaminated
Cocaine: An Emerging Public Health Challenge”, Mayo
Clinic Proceedings, vol. 87, issue 6, June. Available online
at: https://www.mayoclinicproceedings.org/article/S0025-
6196(12)00390-4/pdf (accessed 15 May 2020).
Karch, S. (1996), “Cardiac arrest in cocaine users”, The
American Journal of Emergency Medicine, vol. 14, issue
1, January.
COCAINE INSIGHTS

45
Karch, S. (ed.) (2008), Pharmacokinetics and Pharmacody-
namics of Abused Drugs, CRC Press, Boca Raton.
Karch, S., Mari, F., Bartolini, V. and Bertol, E. (2012), “Ami-
norex poisoning in cocaine abusers”, International Journal
of Cardiology, vol. 158, issue 3, July.
Karch, S., Defraia, B., Messerini, L., Mari, F., Vaiano, F. and
Bertol, E. (2014), “Aminorex associated with possible idio-
pathic pulmonary hypertension in a cocaine user”, Forensic
Science International, vol. 240, July.
Karch, S. and Drummer, O. (2015), Karch’s Pathology of
drug abuse, 5th Edition, CRC Press, Boca Raton.
Kenyon, S., Ramsey, J., Lee, T., Johnston, A. and Holt, D.
(2005), “Analysis for Identification in Amnesty Bin Samples
from Dance Venues”, Therapeutic Drug Monitoring, vol.
27, issue 6, December.
Kilmer, B. and Hoorens, S. (eds.) (2010), Understanding
illicit drug markets, supply-reduction efforts, and drug-
related crime in the European Union, report prepared
for the European Commission, DG Justice, Freedom
and Security, RAND Europe, Brussels. Available online
at: https://www.rand.org/pubs/technical_reports/TR755.
html (accessed 15 June 2020).
King, L. (1997), “Drug content of powders and other illicit
preparations in the UK”, Forensic Science International,
vol. 85 issue 2, February.
Klein, A., Day, M., Harriot, A. (eds.) (2004), Caribbean
Drugs, Zed Books, London.
Knowles, L., Buxton, J., Skuridina, N., Achebe, I.,
LeGatt, D., Fan, S., Zhu, N. and Talbot, J. (2009),
“Levamisole tainted cocaine causing severe neutrope-
nia in Alberta and British Columbia”, Harm Reduction
Journal, vol. 6, issue 30, November. Available online
at: https://harmreductionjournal.biomedcentral.com/
track/pdf/10.1186/1477-7517-6-30 (accessed 1 May
2020).
Knuth, M., Temme, O., Daldrup, T. and Pawlik, E. (2018),
“Analysis of cocaine adulterants in human brain in cases
of drug-related death”, Forensic Science International,
vol. 285, April.
Kudlacek, O., Hofmaier, T., Luf, A., Mayer, F. P., Stockner, T.,
Nagy, C., Holy, M., Freissmuth, M., Schmid, L. and Sitte,
H. (2017), “Cocaine adulteration”, Journal of Chemical
Neuroanatomy, vol. 83-84, October.
Laniel, L. (2017), “Captagon. Déconstruction d’un mythe”,
Drogues, enjeux internationaux, n° 10, July. Available
online at: https://www.ofdt.fr/index.php?cID=939 (accessed
18 July 2020).
Lapachinske, S., Okai, G., dos Santos, A., de Bairros, A.
and Yonamine, M. (2015), “Analysis of cocaine and its
adulterants in drugs for international trafficking seized
by the Brazilian Federal Police”. Forensic Science Interna-
tional, vol. 247, February.
Larocque, A. and Hoffman, R. (2012), “Levamisole in
cocaine: Unexpected news from an old acquaintance”,
Clinical Toxicology, vol. 50, issue 4, March.
Lee, D. (1976), The Cocaine Consumer Handbook, And/
Or Press, Berkeley.
LeGatt, D., Boisvert, Y. and Colbourne, P. (2007), “Cocaine
and hog de-wormer: an interesting combination”, Thera-
peutic Drug Monitoring, vol. 29, issue 4, August.
Leggett, T. (2002), Rainbow Vice. The Drugs and Sex Indus-
tries in the New South Africa, Zed Books, London.
Lizasoain, I., Moro, M. and Lorenzo, P. (2002) “Cocaína:
aspectos farmacológicos”, Adicciones, vol. 14, n° 1. Avail-
able online at: http://adicciones.es/index.php/adicciones/
article/view/513/508 (accessed 14 april 2020).
Lociciro, S., Esseiva, P., Hayoz, P., Dujourdy, L., Besacier,
F. and Margot, P. (2008), “Cocaine profiling for strategic
intelligence, a cross-border project between France and
Switzerland”. Forensic Science International, vol. 177,
issues 2-3, May.
López-Hill, X., Prieto, J., Meikle, M., Urbanavicius, J., Abin-
Carriquiry, J. Prunell, G., Umpiérrez, E. Scorza, M., (2011),
“Coca-paste seized samples characterization: Chemical
analysis, stimulating effect in rats and relevance of caf-
feine as a major adulterant”, Behavioural Brain Research,
vol. 11, issue 1, August.
Magalhães, E., Nascentes, C., Pereira, L., Guedes, M., Lor-
deiro, R., Auler, L., Augusti, R. and de Queiroz, M. (2013),
“Evaluation of the composition of street cocaine seized
in two regions of Brazil”, Science & Justice, vol. 53, issue
4, December.
Maghsoudi, N., McDonald, K., Stefan, C. et al. (2020),
Evaluating networked drug checking services in Toronto,
Ontario: study protocol and rationale, Harm Reduction
Journal, vol. 17, issue 9, January. Available online at:
https://harmreductionjournal.biomedcentral.com/arti-
cles/10.1186/s12954-019-0336-0 (accessed 15 May 2020).
Maietti, S., Castagna, F., Molin, L., Ferrara, S. and Traldi, P.
(2009), “Cocaine adulterants used as marker compounds”,
Journal of Mass Spectrometry, vol. 44, issue 7, March.
Maldaner, A., Botelho, E., Zacca, J., Melo, R., Costa, J., Zan-
canaro, Oliveira, C., Kasakoff, L. Paixão, T. (2016), “Chemical
Profiling of Street Cocaine from Different Brazilian Regions”,
Volume 2

46
Journal of the Brazilian Chemical Society, vol. 27, n° 4.
Mallette, J. and Casale, J. (2014), “Rapid determination of
the isomeric truxillines in illicit cocaine via capillary gas
chromatography/flame ionization detection and their use
and implication in the determination of cocaine origin
and trafficking routes”, Journal of Chromatography A, vol.
1364, 17 October.
Mallette, J., Casale, J., Colley, Morello, D. and Jordan, J.
(2018), “Changes in illicit cocaine hydrochloride process-
ing identified and revealed through multivariate analysis
of cocaine signature data”, Justice & Science, vol. 58, n°
2, March.
Mallette, J., Casale, J. and Jones, L. (2013), “The
Separation of Cocaine and Phenyltetrahydroimidazo-
thiazole Mixtures”, Microgram, vol. 10, n° 2. Available
online at: https://www.dea.gov/sites/default/files/pr/
microgram-journals/2013/mj10-1_12-16.pdf (accessed
23 April 2020).
Malpica, K. (n.d.), “Crack”, Las drogas tal cual, webpage.
Available online at: https://www.mind-surf.net/drogas/
crack.htm (accessed 20 July 2020).
Manschreck, T., Laughery, J., Weisstein, C., Allen, D.,
Humblestone, B., Neville, M., Podlewski, H. and Mitra,
N. (1988), “Characteristics of Freebase Cocaine Psychosis”,
Yale Journal of Biology and Medicine, vol. 61, n° 2.
Marcelo, M., Mariotti, K., Ferrao, M. and Ortiz, R. (2015),
“Profiling cocaine by ATR–FTIR” Forensic Science Inter-
national, vol. 246, January.
Marcelo, M., Mariotti, K., Ortiz, R., Ferrao, M. and
Anzanello, M. (2016), “Scott Test Evaluation by Multivari-
ate Image Analysis in Cocaine Samples”, Microchemical
Journal, vol. 127, July.
Martello, S., Pieri, M., Ialongo, C., Pignalosa, S., Noce, G.,
Vernich, F., Russo, C., Mineo, F., Bernardini, S. and Mar-
sella, L. T. (2017), “Levamisole in Illicit Trafficking Cocaine
Seized: A One-Year Study”, Journal of Psychoactive Drugs,
vol. 49, issue 5.
McDonald, K., Maghsoudi, N., Thompson, H. and Werb,
D. (2020), What’s in Toronto’s Drug Supply? Results from
Samples Checked by Toronto’s Drug Checking Service
October 10, 2019 - March 31, 2020, Centre on Drug Policy
Evaluation, Toronto, 14 April. Available online at: http://
cdpe.org/wp-content/uploads/2020/04/DCS-Report_
Oct2019-Mar2020_final.pdf (accessed 15 May 2020).
McGill, J., Dixon, C., and Ritter, D. (2008), “Discovery of
an Interesting Temperature Effect on the Sensitivity of the
Cobalt Thiocyanate Test for Cocaine”, Microgram Journal,
vol. 6, n° 1-2, January – June.
Medeiros, R., Filho, N. and Leles, M. (2009), “Development
of forensic analytical chemistry method for examination of
Merla by thermal analysis and high resolution gas chro-
matography”, Journal of Thermal Analysis and Calorimetry,
vol. 97, n° 337.
Míguez, H. (2008), “Prevalencia del uso de pasta base y
riesgo social”, Vertex, vol. XIX, n° 77, January-March.
Mingardi, G. and Goulart, S. (2002), “Drug Trafficking
in an Urban area: The case of São Paulo”, in in Geffray,
C., Fabre, G. and Schiray, M. (coord.), Drug Trafficking,
Criminal Organisations and Money Laundering, vol. 2,
MOST-UNESCO-UNODCCP, Paris.
Molina, J. (2014), Investigación sobre el bazuco en
Bogotá: componentes, adulterantes y residuos, online
article, Échele Cabeza. Available online at: https://www.
echelecabeza.com/investigacion-sobre-el-bazuco-en-
bogota-componentes-adulterantes-y-residuos/ (accessed
22 July 2020).
Monfreda, M., Varani, F., Cattaruzza, F., Ciambrone, S. and
Proposito, A. (2015), “Fast profiling of cocaine seizures by
FTIR spectroscopy and GC-MS analysis of minor alkaloids
and residual solvents”, Science & Justice, vol. 55, issue
6, December.
Moore, J. and Casale, J. (1994), “In-depth chromatographic
analyses of illicit cocaine and its precursor, coca leaves”,
Journal of Chromatography A, vol. 674, issue 1-2.
Moraes, M. (2015), “Diez años de investigación en pasta
base de cocaína en Uruguay” Archivos de Pediatría del
Uruguay, vol. 85, n° 3. Available online at: https://www.
sup.org.uy/archivos-de-pediatria/adp85-3/web/pdf/
adp85-3_editorial.pdf (accessed 24 July 2020).
Moraes, M., González, G., Castelli, L., Umpiérrez, E. and
Sosa, C. (2015), Consumo de pasta base de cocaína
y cocaína en mujeres durante el embarazo, Espacio
Interdisciplinario de la Universidad de la República, Mon-
tevideo. Available online at: https://www.researchgate.
net/publication/303858823_Consumo_de_pasta_base_
de_cocaina_y_cocaina_en_mujeres_durante_el_embarazo
(accessed 22 July 2020).
Morales-Vaca, M; (1984), “A laboratory approach to the con-
trol of cocaine in Bolivia”, Bulletin on Narcotics, vol. 36, n° 2.
Morelato, M., Franscella, D., Esseiva, P. and Broséus, J.
(2019), “When does the cutting of cocaine and heroin
occur? The first large-scale study based on the chemical
analysis of cocaine and heroin seizures in Switzerland”,
International Journal of Drug Policy, vol. 73.
Morello, D. and Meyers, P. (1995), “Qualitative and quan-
titative determination of residual solvents in illicit cocaine
COCAINE INSIGHTS

47
HCl and heroin HCl”, Journal of Forensic Science, vol. 40,
n° 6, November.
Néfau, T., Charpentier, E., Elyasmino, N., Duplessy-Garson,
C., Levi, Y. and Karolak, S. (2015), “Drug analysis of residual
content of used syringes: A new approach for improving
knowledge of injected drugs and drug user practices”,
International Journal of Drug Policy, vol. 26, issue 4, April.
Neves, O. (2013), Caracterização de amostras de cocaína
apreendidas pela Polícia civil do estado de Rondônia,
Dissertação, Mestrado em Desenvolvimento Regional
e Meio Ambiente, Universidade Federal de Rondônia,
Porto Velho, May. Available online at: https://www.ri.unir.
br/jspui/bitstream/123456789/2233/1/DISSERTAÇÃO%20
GUSTAVO%20DE%20OLIVEIRA%20NEVES.pdf (accessed
7 May 2020).
Neves, O. and Nunes, B. (2008), “Adulterants found in
mixtures of illegal psychoactive drugs”, Revista da Facul-
dade de Ciências da Saúde, vol. 5.
NIDA (2016), Cocaine, Research Reports Series, National
Institute on Drug Abuse, May. Available online at: https://
www.drugabuse.gov/node/pdf/1141/cocaine (accessed 29
April 2020)
ODC (2017), Reporte de Drogas de Colombia 2017, Obser-
vatorio de Drogas de Colombia, Ministerio de Justicia y del
Derecho, Bogotá, October. Available online at: http://www.
odc.gov.co/PUBLICACIONES/ArtMID/4214/ArticleID/5693/
Reporte-de-Drogas-de-Colombia-2017 (accessed 21 July
2020).
OFDT (2012), Cocaïne, données essentielles, Observatoire
français des drogues et des toxicomanies, Saint-Denis,
March. Available online at : https://www.ofdt.fr/BDD/pub-
lications/docs/codescomp.pdf (accessed 6 April 2020)
OFDT (2013), La cocaïne basée en France métropolitaine :
évolutions récentes, Observatoire français des drogues et
des toxicomanies, Saint-Denis, December. Available online
at : https://www.ofdt.fr/BDD/publications/docs/eftxmgtc.
pdf (accessed 13 April 2020).
OFDT (2018), Usages et ventes de crack à Paris. Un état
des lieux 2012-2017, Observatoire français des drogues
et des toxicomanies, Saint-Denis, March. Available online
at : https://www.ofdt.fr/BDD/publications/docs/epfxacy3.
pdf (accessed 17 April 2020).
OGD (1996), Atlas mondial des drogues, Observatoire géo-
politique des drogues, Presses universitaires de France,
Paris.
OGD (1998), The World Geopolitics of Drugs 1997/1998,
annual report, Observatoire géopolitique des drogues,
Paris, October. Available online at: http://www.
mamacoca.org/docs_de_base/Cifras_cuadro_mamacoca/
OGD_chez_mamacoca/OGD_The_World_Geopolitics_of_
Drugs_1997-1998.pdf (accessed 16 April 2020).
O’Rourke, P.J. (1991), Parliament of Whores, Picador,
London.
OUD (2014), Pasta base de cocaína en Uruguay. Compi-
lación, Observatorio uruguayo de drogas, Montevideo,
February. Available online at: https://www.gub.uy/junta-
nacional-drogas/sites/junta-nacional-drogas/files/2018-01/
Pasta_Base_en_Uruguay_Compilacion_0.pdf (accessed
12 April 2020).
Pascale, A., Negrin, A. and Laborde, A. (2010), “Pasta base
de cocaína: experiencia del Centro de Información y Ase-
soramiento Toxicológico”, Adicciones, vol. 22, n° 3.
Pawlik, E. and Mahler, H. (2011), “Smoke analysis of adul-
terated illicit drug preparations”, Toxichem Krimtech, vol.
78, special issue, April. Available online at: https://www.
gtfch.org/cms/images/stories/media/tb/tb2011/pawlik.pdf
(accessed 28 May 2020).
Pawlik, E., Mahler, H., Hartung, B., Plässer, G. and Daldrup,
T. (2015), “Drug-related death: Adulterants from cocaine
preparations in lung tissue and blood”, Forensic Science
International, vol. 249, April.
Payer, D., Young, M., Maloney-Hall, B., Mill, C., Leclerc,
P., Buxton, J., the Canadian Community Epidemiology
Network on Drug Use and the National Drug Checking
Working Group (2020), Adulterants, contaminants and
co-occurring substances in drugs on the illegal market
in Canada: An analysis of data from drug seizures, drug
checking and urine toxicology, Canadian Centre on Sub-
stance Use and Addiction, Ottawa, April. Available online
at: https://www.ccsa.ca/sites/default/files/2020-04/CCSA-
CCENDU-Adulterants-Contaminants-Co-occurring-Sub-
stances-in-Drugs-Canada-Report-2020-en.pdf (accessed
21 April 2020).
Perez-Reyes, M., Di Guiseppi, S., Ondrusek, G., Jeffcoat, A.,
and Cook, C. (1982), “Free-base cocaine smoking”, Clinical
Pharmacology and Therapeutics, vol. 32 n° 4.
Perry, C. (1980), “Freebase: A Treacherous Obsession”,
Rolling Stone, 1 May. Available online at: https://www.
rollingstone.com/culture/culture-news/freebase-a-treach-
erous-obsession-73987/ (accessed 5 April 2020).
Pilgrim, J., Woodford, N. and Drummer, O. (2013), “Cocaine
in sudden and unexpected death: A review of 49 post-
mortem cases”, Forensic Science International, vol. 227,
issues 1-3, April.
Plowman, T. (1981), “Amazonian Coca”, Journal of Ethno-
pharmacology, vol. 3.
Volume 2

48
Pope, J., Drummer, O. and Schneider, H. (2018), “The
cocaine cutting agent levamisole is frequently detected
in cocaine users”. Pathology, vol. 50, issue 5, August.
Prieto, J. and Scorza, C. (2010), Pasta base de cocaína,
articulo de divulgación (Uruguay). Available online at:
http://www.iibce.edu.uy/DIVULGACION/Articulo%20
de%20divulgacion%20de%20Uruguay-%20PASTA%20
BASE%20DE%20COCAINA.pdf (accessed 24 July 2020).
Prieto, J., Galvalisi, M., López-Hill, X. Meikle, M., Abin-
Carriquiry, J. and Scorza, C. (2015), “Caffeine Enhances
and Accelerates the Expression of Sensitization Induced by
Coca Paste Indicating its Relevance as a Main Adulterant”,
The American Journal on Addictions, vol. 24.
Ralón, G., Rossi, D., Vila, M., Latorre, L., Bastos, F. and
Caiaffa, W. (2012), “De los estudios locales a una perspec-
tiva regional: análisis integrado de datos secundarios en un
proyecto colaborativo sobre vulnerabilidades asociadas al
uso de drogas en Argentina, Brasil y Uruguay (1998-2004)”,
Salud Colectiva, vol. 8, n° 3, September-December. Avail-
able online at: http://intercambios.org.ar/publicaciones/
Ralón%20et%20al.%20Salud%20Colectiva%202014%20
en%20español.pdf (accessed 2 July 2020).
Raymon, L. and Isenschmid, D. (2009), “The Possible Role
of Levamisole in Illicit Cocaine Preparations”, Journal of
Analytical Toxicology, vol. 33, n° 9.
Ragoucy-Sengler, C., Simonetti, M. and Kintz, P. (2003),
“Cocaïne chlorhydrate et cocaïne base ou crack : quelles
différences ?”, Psychotropes, vol. 9, 2003/2.
Reuter, P. and Caulkins, J. (2004), “Illegal ‘lemons’: price
dispersion in cocaine and heroin markets”, Bulletin on
Narcotics, vol. LVI, n° 1 & 2. Available online at: https://
www.unodc.org/pdf/bulletin/bulletin_2004_01_01_1.pdf
(accessed 27 April 2020).
Release (2020), “Crack cocaine”. Release–Drugs, the Law
and Human Rights. Available online at: https://www.
release.org.uk/drugs/crack-cocaine/description (accessed
22 July 2021)
Reynaud-Maurupt, C. (2012), Repérage, Prévention et
Réduction des risques de l’usage de cocaïne basée. Revue
de la littérature, Groupe de Recherche sur la Vulnérabil-
ité Sociale, CIRDD-Bretagne, June. Available online at :
http://www.grvs06.org/doc/Revue.Litterature.CC.basee.
JUIN2012.pdf (accessed 1 May 2020).
Ribeiro, M. (2012), “Desmistificando o ‘oxi’: análise
química do perfil dessa ‘nova droga brasileira’ oriunda
do Estado do Acre”, pdf document, Unidade de Pesquisa
em Álcool e Drogas (UNIAD), São Paulo. Available online
at: https://www.uniad.org.br/wp-content/uploads/2012/05/
Artigo_Desmistificando_o_oxi.pdf (accessed 15 July 2020).
Ribeiro de Araújo, M., Trevizol, A., Frajzinger, R., Ribeiro,
A., Speierl, H., Pires, L., Andraus, M., Tsanaclis, L., Sala
Alonso, A., Cordeiro, Q. and Laranjeira, R. (2019), “Adulter-
ants in crack cocaine in Brazil”, Trends in Psychiatry and
Psychotherapy, vol. 41, issue 2.
Rivier, L. (1981), “Analysis of Alkaloids in Leaves of Cul-
tivated Erythroxylum and Characterization of Alkaline
Substances Used During Coca Chewing”, Journal of Eth-
nopharmacology, vol. 3, issues 2-3, March-May.
Rodriguez, W. and Allred, R. (2005), “Synthesis of trans-
4-Methylaminorex from Norephedrine and Potassium
Cyanate”, Microgram Journal, vol. 3, n° 3-4, July-December.
Romo-Avilés, N., Camarotti A., Tarragona A. and Touris,
C. (2015), “Doing gender in a toxic world. Women and
freebase cocaine in the city of Buenos Aires (Argentina)”,
Substance Use & Misuse, vol. 50, issue 5.
Sabogal, J. and Urrego, J. (2012), “Composición química
de muestras de bazuco incautado en Colombia primer
semestre de 2010”, vol. 14, n° 6. Available online at : https://
revistas.unal.edu.co/index.php/revsaludpublica/article/
view/34766/42705 (accessed 21
st
July 2020).
SAMSHA (2019), Key substance use and mental health
indicators in the United States: Results from the 2018
National Survey on Drug Use and Health Substance Abuse
and Mental Health Services Administration, Substance
Abuse and Mental Health Services Administration, Rock-
ville, MD, August. Available online at: https://www.samhsa.
gov/data/sites/default/files/cbhsq-reports/NSDUHNational-
FindingsReport2018/NSDUHNationalFindingsReport2018.
pdf (accessed 26 April 2020)
Sant’Ana, L., de Sousa, V., dos Santos, F., Sabinoa, B.,
Cardoso, A., de Lima, M. and Castro, R. (2019), “Evaluation
of cocaine samples seized in the streets of the state of Rio
de Janeiro, Brazil”, Química nova, vol. 42, n°4.
Santis, R., Hidalgo, C., Hayden, V., Anselmo, E., Rodrí-
guez, J., Cartajena de la M, F. Dreyse, J., Torre, R. (2007),
“Consumo de sustancias y conductas de riesgo en consum-
idores de pasta base de cocaína y clorhidrato de cocaína
no consultantes a servicios de rehabilitación”, Revista
Médica de Chile, vol. 135, n° 1, January. Available online
at: https://scielo.conicyt.cl/scielo.php?script=sci_arttext&
pid=S0034-98872007000100007 (Accessed 14 April 2020).
Schlesinger, H. (1985), “Topics in the chemistry of cocaine”,
Bulletin on Narcotics, vol. XXXVII, n° 1. Available online
at: https://www.unodc.org/unodc/en/data-and-analysis/
bulletin/bulletin_1985-01-01_1_page006.html (accessed
29 April 2020).
Schneider, S. and Meys, F. (2011), “Analysis of illicit cocaine
and heroin samples seized in Luxembourg from 2005–2010”,
COCAINE INSIGHTS

49
Forensic Science International, vol. 212, issues 1-3, October.
Sedronar (2007), Aspectos cualitativos del consumo de
Pasta Base de Cocaína/Paco, Secretaría de Políticas Inte-
grales sobre Drogas de la Nación Argentina, Buenos Aires,
September.
Sedronar (2015), Caracterización Química de las Cocaínas
Fumables, Secretaría de Políticas Integrales sobre Drogas
de la Nación Argentina, Buenos Aires. Available online at
: http://www.observatorio.gov.ar/media/k2/attachments/
CaracterizacinZQumicaZdeZlasZCocanasZFumablesZ-
ZAosZ2014Z-Z2015.pdf (accessed 20 July 2020).
Sedronar (2019), Boletín estadístico del perfil de pacien-
tes asistidos, transferencias por becas de tratamiento
y llamadas al servicio de atención de la línea 141, 3°
trimestre de 2019, Observatorio Argentino de Drogas, Sec-
retaría de Políticas Integrales sobre Drogas de la Nación
Argentina. Available online at : http://www.observatorio.
gov.ar/media/k2/attachments/TercerZtrimestreZ2019.pdf
(accessed 12 April 2020).
Shannon, M. (1988), “Clinical toxicity of cocaine adulterants”,
Annals of Emergency Medicine, vol. 17, issue 11, November.
Shannon, M., Borron, S. and Burns, M. (eds) (2007),
Haddad and Winchester’s clinical management of poi-
soning and drug overdose, 4th Edition, Saunders Elsevier,
Philadelphia.
Shearer, J., Johnston, J., Kaye, S., Collins, L. and Dillon, P.
(2005), “Characteristics and Dynamics of Cocaine Supply
and Demand in Sydney and Melbourne”, National Drug
Law Enforcement Research Fund, Monograph Series No.
14, Adelaide.
Shesser, R., Jotte, R. and Olshaker, J. (1991), “The contri-
bution of impurities to the acute morbidity of illegal drug
use”, The American Journal of Emergency Medicine, vol.
9, issue 4.
Sidiguitiebe, C. (2016), “Un laboratoire de fabrication
de cocaïne démantelé à Oujda”, TelQuel, 9 September.
Available online at: https://telquel.ma/2016/09/09/labo-
fabrication-cocaine-demantele-oujda_1513801 (accessed
18 April 2020).
Siegel, R. (1982), “Cocaine Free Base Use”, Journal of Psy-
choactive Drugs, vol. 24, issue 2. SIMCI (2019a), Colombia.
Monitoreo de territorios afectados por cultivos ilícitos,
Sistema Integrado de Monitoreo de Cultivos Ilícitos,
United Nations Office on Drugs and Crime, Bogotá.
SIMCI (2019b), Colombia. Producción ilícita de drogas:
estudios sobre drogas naturales, sintéticas y NPS, Sistema
Integrado de Monitoreo de Cultivos Ilícitos, United Nations
Office on Drugs and Crime, Bogotá.
Soine, W. (1986), “Clandestine Drug Synthesis”, Medicinal
Research Reviews, vol. 6, n° 1, January-March.
Solimini, R., Rotolo, M., Pellegrini, M., Minutillo, A., Paci-
fici, R., Busardò, F. and Zaami, S. (2017), “Adulteration
Practices of Psychoactive Illicit Drugs: An Updated Review”,
Current Pharmaceutical Biotechnology, vol. 18, n° 7.
Solomon, N. and Hayes, J. (2017), “Levamisole: A High
Performance Cutting Agent”, Academic Forensic Pathol-
ogy, vol. 7, issue 3, September.
Stambouli, H. and El Bouri, A. (2017), “Chemical Profile
of Cocaine Seizures and Its Adulterants in Morocco”,
Forensic Science Addiction Research, vol. 1, issue 4. Avail-
able online at: https://crimsonpublishers.com/fsar/pdf/
FSAR.000520.pdf (accessed 7 May 2020).
Stinus, L. (1992), “La dépendance à la cocaine”, La Revue
du praticien-Médecine Générale, n° 179, 18 May.
Stride Nielsen, L., Villesen, P. and Lindholst, C. (2016),
“Stability of cocaine impurity profiles during 12 months
of storage”, Forensic Science International, vol. 264, July.
Stride Nielsen, L., Villesen, P. and Lindholst, C. (2017), “Vari-
ation in chemical profiles within large seizures of cocaine
bricks”, Forensic Science International, 280, November.
Suárez, H., Ramírez, J., Albano, G., Castelli, L., Martínez,
E. and Rossal, M. (2014), Fisuras. Dos estudios sobre
pasta base de cocaína en el Uruguay. Aproximaciones
cuantitativas y etnográficas, Universidad de la República,
Montevideo. Available online at : https://www.gub.uy/
junta-nacional-drogas/comunicacion/publicaciones/
fisuras-dos-estudios-sobre-pasta-base-cocaina-uruguay-
ano-2014 (accessed 11 April 2020).
TEDI (n.d.), TEDI Trend Report, TEDI Trend Reports 1 to
4 (2011-2013), Trans European Drug Information proj-
ect, Available online at: https://webgate.ec.europa.eu/
chafea_pdb/assets/files/pdb/20101207/20101207_d06-02_
en_psbinder.pdf (accessed 14 May 2020).
Ti, L., Buxton, J., Wood, E., Zhang, R., Montaner, J. and
Kerr, T., (2011) “Difficulty accessing crack pipes and crack
pipe sharing among people who use drugs in Vancouver,
Canada”, Substance Abuse Treatment, Prevention, and
Policy, vol. 6, December.
TNI (2006), El paco bajo la lupa. El mercado de la pasta base
de cocaína en el Cono Sur, TNI Briefing Series, n° 2006/4,
Transnational Institute, Amsterdam. Available online at:
https://www.tni.org/files/download/200612281211405043.
pdf (accessed 19 July 2020).
TNI (2019), Smokable cocaine markets in Latin America
and the Caribbean, Transnational Institute, Amsterdam,
Volume 2

50
December. Available online at: https://www.tni.org/files/
publication-downloads/tni-smokablecocaine_eng_web-def.
pdf (accessed 11 April 2020).
Trimbos Instituut (2016), Annual Report 2015. Drugs Infor-
mation and Monitoring System (DIMS), Utrecht. Available
online at: https://www.trimbos.nl/docs/9fd7322f-fc0b-46b1-
8643-591b91cc33a5.pdf (accessed 17 May 2020).
Trimbos Instituut (2019), Annual Report 2018. Drugs Infor-
mation and Monitoring System (DIMS), Utrecht. Available
online at: https://www.trimbos.nl/docs/2874f3d0-7355-
4d41-9480-00e9dced5fa6.pdf (accessed 17 May 2020).
UNODC (2005), Methods for Impurity Profiling of Heroin
and Cocaine. Manual for Use by National Drug Testing
Laboratories, United Nations Office on Drugs and Crime,
Laboratory and Scientific Section, Vienna. Available online
at: https://www.unodc.org/documents/publications/report_
st-nar-35.pdf (accessed 10 April 2020).
UNODC (2012), Recommended methods for the Identifica-
tion and Analysis of Cocaine in Seized Materials, United
Nations Office on Drugs and Crime, Laboratory and Sci-
entific Section, Vienna, March. Available online at: https://
www.unodc.org/documents/scientific/Cocaine_Manual_
Rev_1.pdf (accessed 24 April 2020).
UNODC (2013), Pasta Básica de Cocaína. Cuatro déca-
das de historia, actualidad y desafíos, United Nations
Office on Drugs and Crime and Comisión Nacional para
el Desarrolla y Vida sin Drogas (DEVIDA), Lima. Available
online at: https://www.unodc.org/documents/peruandec-
uador//Publicaciones/Publicaciones2013/LIBRO_PBC.pdf
(Accessed 11 April 2020).
UNODC (2016a), Terminology and Information on Drugs.
Third Edition (United Nations publication, Sales No. E.16.
XI.8), March. Available online at: https://www.unodc.org/
documents/scientific/Terminology_and_Information_on_
Drugs-E_3rd_edition.pdf (accessed 21 April 2021).
UNODC (2016b), World Drug Report 2016 (United Nations
publication, Sales No. E.16.XI.7). Available online at:
https://www.unodc.org/wdr2016/ (accessed 23 July 2021)
UNODC (2017) Systematic Literature Review on Stimulant
use and HIV (A) - Part 3/5 - Cocaine use and HIV Risk and
Transmission, United Nations Office on Drugs and Crime.
Available online at: https://www.unodc.org/documents/
hiv-aids/2017/3_Stim_HIV_Syst_Lit_Rev_Part_3_Cocaine_
and_Crack-Cocaine.pdf (accessed 23 July 2021).
UNODC (2019a), Treatment of Stimulant Use Disorders:
Current Practices and Promising Perspectives – Discussion
Paper, March. Available online at: https://www.unodc.org/
documents/drug-prevention-and-treatment/Treatment_of_
PSUD_for_website_24.05.19.pdf (accessed 1 July 2021).
UNODC (2019b), World Drug Report 2019 (United
Nations publication, Sales No. E.19.XI.8). Available
online at: https://wdr.unodc.org/wdr2019/ (accessed
24 March 2020).
UNODC (2019c), Global Study on Homicide 2019, United
Nations Office on Drugs and Crime, July. Available
online at: https://www.unodc.org/unodc/en/data-and-
analysis/global-study-on-homicide.html (accessed 23
July 2021).
UNODC (2021a), World Drug Report 2021 (United Nations
publication, Sales No. E.21.XI.8). Available online at:
https://www.unodc.org/unodc/en/data-and-analysis/
wdr2021.html (accessed 1 July 2021).
UNODC and EUROPOL (2021b), The illicit trade of cocaine
from Latin America to Europe – from oligopolies to free-
for-all?, Cocaine Insights 1, UNODC, Vienna, September.
Available online at: https://www.unodc.org/unodc/en/data-
and-analysis/the-cocaine-market.html
UNODC and OAS (2014), Amphetamine-Type Stimulants
in Latin America 2014, Global SMART Programme, United
Nations Office on Drugs and Crime and Organisation of
American States, February.
Valentino, A. and Fuentecilla, K. (2005), “Levamisole:
An analytical profile”, Microgram Journal, vol. 3, n° 3-4,
July-December.
Ventura, M., Caudevilla, F. and Vidal, C. (2011), “Cocaína
adulterada con levamisol: posibles implicaciones clínicas”,
Medicina Clínica, vol. 136, issue 8, March. Available online
at : https://energycontrol-international.org/wp-content/
uploads/2016/04/Coca%C3%ADna-adulterada-con-levami-
sol-posibles-implicaciones-cl%C3%ADnicas.pdf (accessed
20 April 2020).
Verri, P., Rustichelli, C., Ferrari, A., Marchesi, P., Baraldi,
C., Licata, M., Vandelli, D., Palazzoli, F., Potì, F. and Enrico
Silingar (2019), “Seizures of illicit substances for personal
use in two Italian provinces: analysis of trends by type
and purity from 2008 to 2017”, Substance Abuse Treatment,
Prevention, and Policy, vol. 14, art. N° 41, September.
Available online at: https://substanceabusepolicy.biomed-
central.com/articles/10.1186/s13011-019-0229-y (accessed
25 April 2020).
Viana, N. (2005), “Oxi: A New Drug in the Amazon”, The
Narco News Bulletin, issue 37, 13 May. Available online
at: http://www.narconews.com/Issue37/article1288.html
(accessed 22 June 2020).
Vidal, C., Fornís, I. and Ventura, M. (2014), “New psycho-
active substances as adulterants of controlled drugs. A
worrying phenomenon?”, Drug Testing and Analysis, vol.
6, issues 7-8.
COCAINE INSIGHTS

51
Vieira Duarte, P. de Andrade Stempliuk, V. and Pereira
Barroso, L. (orgs.) (2009), Relatório brasileiro sobre
drogas, Secretaria Nacional de Políticas sobre Drogas
(SENAD), Brasilia. Available online at : https://www.jus-
tica.gov.br/central-de-conteudo/politicas-sobre-drogas/
relatorios-politicas-sobre-drogas/relatoriobrasileiroso-
bredrogas-2010.pdf (accessed 7 May 2020).
Villar, M., Sánchez, J. and Ruíz, M. (2018), “Purity and
adulteration in cocaine seizures and drug market inspec-
tion in Galicia (Spain) across an eight-year period”, Drug
Testing and Analysis, vol. 10, issue 2, February.
Wexler, P. (ed.) (2014), Encyclopedia of Toxicology, 3rd
Edition, Academic Press, London.
WHO (1994), Glosario de términos de alcohol y drogas,
World Health Organisation. Available online at: https://
www.who.int/substance_abuse/terminology/lexicon_alco-
hol_drugs_spanish.pdf?ua=1 (accessed 21st July 2020).
WHO and UNICRI (1995), The Natural History of
Cocaine Abuse: a case study endeavour, World Health
Organisation and United Nations Interregional Crime
and Research Institute, unpublished, September.
Available online at: https://www.tni.org/files/article-down-
loads/200703081415045872_0.pdf (accessed 12 April 2020).
Zacca, J., Botelho, E., Vieira, M., Almeida, F., Ferreira, L.
and Maldaner, A. (2014), “Brazilian Federal Police drug
chemical profiling — The PeQui Project”, Science & Justice,
vol. 54, issue 4, July. Available online at: https://www.
researchgate.net/publication/260804181_Brazilian_Fed-
eral_Police_drug_chemical_profiling_-_The_PeQui_Project
(accessed 18 May 2020).
Zhu, N. Le Gatt, D. and Turner, R. (2009), “Agranulocy-
tosis after Consumption of Cocaine Adulterated with
Levamisole”, Annals of Internal Medicine, vol. 150, issue
4, February. Available online at: https://www.researchgate.
net/publication/23801121_Agranulocytosis_After_Con-
sumption_of_Cocaine_Adulterated_With_Levamisole
(accessed 8 May 2020).
Volume 2

CRIMJUST is implemented by UNODC in partnership with INTERPOL and
Transparency International. CRIMJUST seeks to enhance law enforce-
ment and judicial strategies beyond interdiction activities and to foster
transnational responses along drug trafficking routes targeting each stage
of the drug supply chain. This includes the production of knowledge on the
cocaine market to support evidence-based policy and strategies designed
to counter the cocaine threat.
Going Beyond Drug Seizures
