
ORNL/TM-2017/382
Evaluation of the Impacts of Heat
Exchanger Operation on Quality of
Water Used as Heat Source and Sink
Ellen D. Smith
Xiaobing Liu
June 30, 2018
Approved for public release.
Distribution is unlimited.

DOCUMENT AVAILABILITY
Reports produced after January 1, 1996, are generally available free via US Department of Energy
(DOE) SciTech Connect.
Website www.osti.gov
Reports produced before January 1, 1996, may be purchased by members of the public from the
following source:
National Technical Information Service
5285 Port Royal Road
Springfield, VA 22161
Telephone 703-605-6000 (1-800-553-6847)
TDD 703-487-4639
Fax 703-605-6900
Website http://classic.ntis.gov/
Reports are available to DOE employees, DOE contractors, Energy Technology Data Exchange
representatives, and International Nuclear Information System representatives from the following
source:
Office of Scientific and Technical Information
PO Box 62
Oak Ridge, TN 37831
Telephone 865-576-8401
Fax 865-576-5728
Website http://www.osti.gov/contact.html
This report was prepared as an account of work sponsored by an
agency of the United States Government. Neither the United States
Government nor any agency thereof, nor any of their employees,
makes any warranty, express or implied, or assumes any legal liability
or responsibility for the accuracy, completeness, or usefulness of any
information, apparatus, product, or process disclosed, or represents
that its use would not infringe privately owned rights. Reference herein
to any specific commercial product, process, or service by trade name,
trademark, manufacturer, or otherwise, does not necessarily
constitute or imply its endorsement, recommendation, or favoring by
the United States Government or any agency thereof. The views and
opinions of authors expressed herein do not necessarily state or reflect
those of the United States Government or any agency thereof.
ORNL/TM-2017/382
EVALUATION OF THE IMPACTS OF HEAT EXCHANGER OPERATION ON QUALITY OF
WATER USED AS HEAT SOURCE AND SINK
Ellen D. Smith
Environmental Sciences Division
Xiaobing Liu
Energy & Transportation Science Division
Date Published: June 30, 2018
Prepared by
OAK RIDGE NATIONAL LABORATORY
Oak Ridge, Tennessee 37831-6283
managed by
UT-BATTELLE, LLC
for the
US DEPARTMENT OF ENERGY
under contract DE-AC05-00OR22725
iii
CONTENTS
Page
LIST OF FIGURES ...................................................................................................................................... v
LIST OF TABLES ........................................................................................................................................ v
ACRONYMS AND ABBREVIATIONS ................................................................................................... v i i
ACKNOWLEDGMENTS ........................................................................................................................... ix
ABSTRACT .................................................................................................................................................. 1
1. BACKGROUND .................................................................................................................................. 1
2. PURPOSE OF THIS INVESTIGATION ............................................................................................. 2
3. DATA PROVIDED FOR REVIEW OF WATER QUALITY IMPACTS OF THE HEAT
EXCHANGER SYSTEM ..................................................................................................................... 3
4. ORNL REVIEW OF ANALYTICAL METHODOLOGY .................................................................. 3
5. ORNL REVIEW OF WATER QUALITY MONITORING RESULTS .............................................. 4
5.1 REGULATORY COMPLIANCE ............................................................................................... 4
5.2 EFFECTS OF THE HEAT EXCHANGER SYSTEM ON WATER QUALITY....................... 5
5.2.1 Water Temperature ........................................................................................................ 5
5.2.2 Biofilms ......................................................................................................................... 6
5.2.2.1 Context for biofilm measurements .................................................................. 6
5.2.2.2 Observations of biofilm formation .................................................................. 7
5.2.2.3 Investigations of Legionella .......................................................................... 11
6. SUMMARY CONCLUSIONS ........................................................................................................... 12
7. REFERENCES ....................................................
............................................................................... 14
APPENDIX A. SUMMARY LIST OF ANALYTES REPORTED TO ORNL BY NYAW ................... A-1
APPENDIX B. METHODOLOGY DESCRIPTIONS PROVIDED TO ORNL BY NYAW .................. B-1
v
LIST OF FIGURES
Page
Figure 1. ATP accumulation rates (mean of two samples) for biofilms vs. mean temperature
recorded in sampling events in 2015 and 2016 during the period when the biofilm was
forming. ................................................................................................................................... 11
LIST OF TABLES
Page
Table 1. Summary statistics for all reported metrics of biofilm formation in inflow and outflow
water lines ................................................................................................................................. 8
Table 2. HPC bacterial densities determined from biofilms formed during the cooling season ................... 9
Table 3. ATP accumulation rates determined from biofilms formed during the cooling season ................ 10
vii
ACRONYMS AND ABBREVIATIONS
ATP adenosine triphosphate
°C degrees Celsius
CFU colony forming uni
t
°F degrees Fahrenhei
t
f
t
feet
ft² square feet
GHP geothermal heat pump
gpm gallons per minute
HPC heterotrophic plate coun
t
MCL maximum contaminant level
mg/L milligrams per lite
r
mL millilite
r
mm² square millimeters
MPN most probable numbe
r
NYAW New York American Wate
r
ORNL Oak Ridge National Laboratory
PCR polymerase chain reaction
qPCR quantitative PCR
pg picograms
VOCs volatile organic compounds
ix
ACKNOWLEDGMENTS
American Water provided financial support for ORNL’s independent review. Project direction and
approval for public release of this report were provided by American Water personnel William Varley,
VP & Deputy COO, Carmen Tierno, President of New York American Water, Benjamin Stanford, Senior
Director of Water Intelligence, and Matt Corson, Director of Environmental Compliance & Stewardship.
Numerous other individuals have contributed to the operation, testing, and analysis of the geothermal heat
pump system at Buck Elementary School, and to writing reports and compiling data. Individuals who
supported the review by providing information to ORNL, discussing the geothermal heat pump system
and the analyses with the authors, and providing comments on drafts of this report included Patrick
Jjemba, Richard Kern, Kendra Morris, and Michael Nofi.
1
ABSTRACT
To help inform future decisions about the subsequent uses of water that has served as a heat source and
sink in the heat exchanger component of a geothermal heat pump system, ORNL performed an
independent assessment of data characterizing the quality of water that had passed through a heat
exchanger supporting a geothermal heat pump in a school building. Water supply to the heat exchanger is
treated drinking water from a public water supply system that derives its water from a groundwater
source. Water analyses and bacteriological measurements collected from heat exchanger inflow and
outflow lines were supplied to ORNL for review. Data were compared with regulatory standards and
inflow and outflow data were compared to identify changes occurring in the water resulting from its
passage through the heat exchanger. Review of the data identified no conditions that would prevent the
use of heat exchange outflow water for water supply. Inflow and outflow water quality conforms with
applicable regulatory standards. There were no discernible differences between inflow and outflow water
quality for any parameters other than water temperature and formation of heterotrophic bacterial biofilms.
Changes in water temperature are an expected result of the operation of the heat exchanger system and do
not by themselves affect the suitability of the water for human consumption or other domestic uses.
Bacterial biofilm formation can be affected by water temperature, but the data did not show a consistent
or statistically significant relationship between temperature parameters and biofilm formation, and the
observations of biofilms formed in the heat exchanger water lines do not affect the suitability of the
outflow water for water supply. Legionella, a bacterial genus that is detected in many different natural
waters and water supplies and that has been associated with outbreaks of waterborne pathogenic disease,
was detected at low levels in some biofilm samples from both inflow and outflow water lines, but
comparison of inflow and outflow data shows that passage of water through the heat exchanger is not
promoting the occurrence of Legionella in this system.
1. BACKGROUND
Geothermal heat pump (GHP) systems, also referred to as ground source heat pump systems, have been
proven capable of producing large reductions in energy use, greenhouse gas emissions and peak period
electricity demand in buildings while satisfying the requirements for space heating, space cooling, and
domestic water heating (DOE 2016). GHPs utilize the ground, groundwater, or surface water as a heat
source and sink. The primary barrier to widespread application of GHP systems is the high installation
cost of the heat source and sink.
Utilizing water from the serving water utility’s water main as the heat source and sink can dramatically
reduce the cost of performance-neutral (or improved) GHP systems by eliminating the need for a
dedicated bore field, thus enabling more widespread application and associated energy and environmental
benefits. Section 3013 of the Energy Policy Act of 1992 directed the Secretary (of Energy) to “encourage
States, municipalities, counties, and townships to consider allowing the installation of geothermal heat
pumps, and, where applicable, and consistent with public health and safety, to permit public and private
water recipients to utilize the flow of water from, and back into, public and private water mains for the
purpose of providing sufficient water supply for the operation of residential and commercial geothermal
heat pumps.”
The purity and safety of the public water supply is the primary mission of water utilities and they are the
entity that would be held responsible if GHP systems utilizing the flow of water from, and back into,
public water mains somehow went awry and contaminated the public water supply. In view of the
potential benefits to consumers and society of this type of system, one water utility installed a pilot
project to assess this technology.
2
In 2014/2015, New York American Water (NYAW), a unit of parent company American Water,
retrofitted William L. Buck Elementary School with a GHP system using water from their water
distribution lines as the heat source and sink. The school is a 40,000 ft² facility constructed in the mid
1950’s in the village of Valley Stream in southwestern Nassau County, New York. NYAW is regulated
by the New York State Public Service Commission. Valley Stream and surrounding communities are
served by NYAW’s Lynbrook Operations District. In this area, the water supply is from groundwater
drawn from wells at depths ranging from 30 to 1,100 ft with an average depth of 500 ft. NYAW
Lynbrook Operations has wells in the Upper Glacial, Magothy, Jameco and Lloyd aquifers. According to
NYAW personnel, water supplied in the area of the school is mostly derived from the Magothy aquifer
(NYAW staff, personal communication in teleconference with ORNL staff on 19 January 2017). The
source water is chlorinated for bacterial disinfection, treated with lime (calcium hydroxide) or caustic
soda (sodium hydroxide) to raise pH and reduce corrosivity, and may also be filtered or treated with
sodium silicate to control dissolved iron (NYAW 2016).
The pilot project at Buck Elementary School was supplied by a 4-inch water service line that delivered
water at rates of 150 to 275 gallons per minute (gpm) to a food-grade stainless steel heat exchanger. On
one side of the heat exchanger is the city water supplied from the water mains; on the other side is a
recirculated mixture of water and propylene glycol that serves heat pumps throughout the school,
providing a combined heating and cooling capacity of 133 tons (Lombardo 2015). The combination of the
city water supply and the heat exchanger eliminated the need for a bore field, significantly reducing the
cost of the GHP system.
In late February 2015 NYAW began operating this system as a pilot in order to collect water quality
samples and record water supply temperature changes. During the pilot phase of this program, after the
supply water passed through the heat exchanger, a portion of the water was discharged back to the aquifer
via a diffusion well and the remainder was discharged to a sanitary sewer. If the water that has passed
through the heat exchanger is found to have acceptable quality, NYAW intends to seek the necessary
approvals to reconfigure the system to recirculate this water in its public water distribution system.
Throughout the pilot phase NYAW monitored water quality in the water lines both upstream and
downstream of the heat exchanger. Water temperature and other key parameters were monitored
continuously by the heat exchanger control system and the full range of required water quality parameters
were analyzed every two weeks. In addition, NYAW conducted the pour plate methodology for detection
of bacterial colonies in water samples (heterotrophic plate counts) and used a coupon system to measure
biofilm formation and detect the presence of Legionella organisms. After Legionella was detected in one
sample cultured from a biofilm, NYAW conducted additional coupon testing for several months and used
a molecular analysis technique to enhance the detection of Legionella, quantify its presence, and identify
any occurrences of the disease-causing species Legionella pneumophila.
2. PURPOSE OF THIS INVESTIGATION
To assist NYAW in evaluating the data from its pilot testing and to help inform future decisions about the
subsequent uses of water that has served as a heat source and sink in the heat exchanger system, Oak
Ridge National Laboratory (ORNL) performed an independent asses
sment of water quality data from the
pilot testing to ascertain the impact of the heat exchanger system on water quality.
3
3. DATA PROVIDED FOR REVIEW OF WATER QUALITY IMPACTS OF THE HEAT
EXCHANGER SYSTEM
NYAW provided data obtained during the pilot phase to ORNL for evaluation and to support an
independent assessment of the impacts of the heat exchanger system on water quality.
Data received were:
1. Tabulated results of field measurements at the time of sample collection of flow rate and inflow and
outflow temperature, free chlorine residual, pH, and pressure. Data were provided for several dates in
early 2015 before the heat exchanger system was fully commissioned and for 41 sampling dates
during full operation, from 19 March 2015 (shortly after the system was commissioned) and 28
September 2016.
2. Reports of results of laboratory analyses of water samples, including total dissolved solids, odor,
turbidity, bacterial counts, hardness, purgeable volatile organic compounds (VOCs), inorganic
compounds, and heterotrophic plate counts. Data were provided for two sampling dates before the
heat exchanger system was fully commissioned and for 41 sampling dates during full operation.
Analyses were performed by Pace Analytical.
3. Tabulated results of measurements of corrosion rates and biofilm formation on mild-steel corrosion
coupons inserted in water lines for periods of approximately one month during the pilot phase testing
of the heat exchanger system. In each test period, two coupons were placed in the inflow line and two
coupons were placed in the outflow line. Twelve sets of data from coupon testing were provided, each
including biofilm data from 4 coupons. For all but two of the test dates, corrosion rates were reported
for both the inflow and outflow location; for two of the twelve testing dates corrosion rates were not
determined for the coupons in the inflow line due to a problem with the testing equipment.
Complete lists of analytical parameters are provided in Appendix A. All of the data received from
NYAW have been archived electronically and can be made available for review.
In addition, NYAW provided ORNL with a report entitled “Additional Evaluation of the Impacts of Heat
Exchanger Operation on Distribution Water Quality” (Jjemba 2018; provided in Appendix B) describing
the results of monitoring conducted in the period May through October 2017, including measurements of
biofilm formation on coupons and molecular analyses to investigate the possible presence of bacteria of
genus Legionella.
4. ORNL REVIEW OF ANALYTICAL METHODOLOGY
The methods used in the analyses provided by NYAW were reviewed to affirm their suitability for the
purposes of this evaluation. Since the main objective of this evaluation is detection of changes in water
quality resulting from passage through the heat exchanger, the most important criterion for acceptability
of methodology is consistency of methodology between inflow and outflow water testing. Other
important considerations include regulatory approval and scientific support for the methods.
Documentation supplied to ORNL indicates that Pace Analytical, which provided laboratory analyses for
NYAW, has appropriate laboratory certifications and the analytical methods identified in the reports
provided to NYAW by Pace Analytical are approved by federal and/or state environmental regulatory
agencies. The Pace Analytical laboratory results reports indicate that samples were handled in accordance
with standard protocols. All water samples were kept cool to the extent practicable and were delivered to
the laboratory on the day of collection. There were no differences in sample handling or analytical
4
methodology for inflow and outflow water samples. For the reasons discussed, the testing laboratory and
its methods are suitable for this investigation.
The data qualifiers in the Pace Analytical results reports were reviewed for indications of data quality
concerns that might affect the interpretability of results. Several of the reports included one or more
notations of a calibration concern affecting a specific analyte. Most of these qualifiers were related to an
organic compound, in which case the qualifier was applied to the analyses of both inflow and outflow
samples. This means that the concern might affect the absolute value of the result for an analyte, but it
should not affect the comparison between inflow and outflow data. Calibration concerns for inorganic
analytes affected analysis of only the inflow or outflow sample, but in all but two instances (an analysis
for barium and an analysis for sulfate) the inorganic analyte was one that was not detected in any sample
during the entire study period. None of the data quality concerns noted in the Pace Analytical results
sheets were judged to have the potential to adversely affect the interpretability of analytical results.
There are no regulatory requirements for analyses of biofilm formation on corrosion coupons or for the
detection of Legionella in samples from potable water systems. NYAW provided a description (NYAW
undated, provided in Appendix B) of the techniques used for the analysis of samples collected in 2015
and 2016. These analyses were performed in the company’s own laboratory in Delran, New Jersey. Mild
steel coupons were inserted in the inflow and outflow water lines for periods of approximately one month,
allowing biofilms to form on the coupons. After the coupons were removed from the water lines, they
were shipped overnight to the laboratory for analysis. At the laboratory, biofilms were scraped off the
coupons and split into several portions for analysis. Rates of biofilm formation during the test period were
determined by an assay of adenosine triphosphate (ATP; a biochemical indicator of biological activity) on
a portion of the biofilm. Another portion of the biofilm was spread plated on R2A agar (Reasoner´s 2A
agar; a formulation for culturing heterotrophic bacteria found in water); colonies were counted after a
one-week incubation. A third portion of the biofilm was cultured and tested for Legionella organisms. As
documented in “NYAWC Geothermal Project Materials and Methods” (NYAW undated), these
methodologies are based on scientific literature and standard methods. The same methods were used for
analyses of inflow and outflow samples. Therefore, these analyses are suitable as a basis for comparing
biofilm formation in inflow and outflow water.
As described above, review of analytical methodologies found that the analyses performed by or for
NYAW were suitable for the review.
5. ORNL REVIEW OF WATER QUALITY MONITORING RESULTS
5.1 REGULATORY COMPLIANCE
Water quality results for both inflow and outflow water were found to meet both U.S. EPA and New York
State regulatory standards for drinking water quality, with the exceptions of several exceedances of
aesthetics-based secondary standards. Exceedances were observed in (1) several measurements for iron
and manganese in both inflow and outflow water and (2) a single event when chlorine odor in both inflow
and outflow water exceeded the threshold for odor. Neither of these exceedances is related to a potential
public health concern and (because they occurred in both inflow and outflow water) neither is attributable
to the heat exchanger. The U.S. EPA drinking water criteria for these parameters are among a set of
nonmandatory secondary standards related to aesthetic considerations (not health). New York State
regulations for public water supplies do include enforceable standards for iron, manganese, and odor, but
review of the regulations indicates that the exceedances of regulatory thresholds reported for heat

5
exchanger inflow and outflow water would not be considered violations of New York State regulations
for public water supplies.
1
5.2 EFFECTS OF THE HEAT EXCHANGER SYSTEM ON WATER QUALITY
Examination of the data from inflow and outflow monitoring found no discernible differences between
inflow and outflow water quality for any parameters other than (1) water temperature and (2) biofilm
formation. Concentrations of dissolved substances, including chlorine residual concentrations, did not
change between inflow and outflow. The two topics of water temperature and biofilm formation are
discussed in the following subsections.
5.2.1 Water Temperature
Water temperature is the only measured water quality parameter that was unmistakably affected by the
operation of the heat exchanger system. Changes in water temperature were an expected result of the use
of water in the system as a heat source and sink.
Additionally, although groundwater sources typically maintain constant temperature throughout the year
(about 55 °F, equivalent to about 13 °C, on Long Island), the temperature of inflow to the heat exchanger
system was observed to vary seasonally. This seasonal variation is attributable to heat gain and loss
within the water distribution system. New York American Water personnel informed ORNL that the
company’s water mains in the Lynnbrook area are typically at depths of about 3 to 4 ft below ground
surface. At this depth, seasonal changes in air temperature can be expected to affect soil temperatures.
Periods of storage in aboveground tanks may also cause water temperatures to begin to equilibrate with
air temperature. Inflow water temperatures reported at the time of sampling events (sampling occurred as
early as 9:00 a.m. and as late as 1:40 pm) ranged from 10.0 °C in June 2017 to 17.6 °C in August 2016.
Operation of the heat exchanger reduces outflow water temperature during heating operation and
increases outflow water temperature during cooling operation. Geothermal heat pump systems can shift
between heating and cooling mode with fluctuations in conditions including outdoor air temperature and
building occupancy, and different modes can exist simultaneously in different parts of a building.
Inspection of temperature data, including records of temperature at 15-minute intervals from the heat-
exchanger control system (supplied by New York American Water for some months of system operation)
indicates that outflow water temperatures were generally lower than inflow temperatures from November
through April, indicating that the system was predominantly operating in heating mode, and were
generally higher than inflow temperatures from June through September, indicating that the system was
predominantly operating in cooling mode. Water temperature data suggest that the months of May and
1
For iron and manganese, the state regulations specify maximum contaminant levels (MCLs) of 0.3 mg/L for each
of these elements and provide that if both iron and manganese are present, the total concentration of both should not
exceed 0.5 mg/L, but higher levels [up to 1.5 mg/L] “may be allowed by the State when justified by the supplier of
water” (New York State Department of Health, Drinking Water Regulations, Part 5, Subpart 5-1, Public Water
Systems – Tables). The highest combined concentration of iron and manganese measured in the heat exchanger
system was 0.94 mg/L. NYAW’s public water quality report (New York American Water, 2016) indicates that the
state has allowed iron and manganese levels in the company’s Lynnbrook Operations to exceed the 0.5-mg/L limit
due to high natural levels of iron and manganese in the system’s water source; treatment measures are employed to
filter out iron and sequester iron that remains in solution to reduce its adverse effects. For odor, a single occurrence
of excessive odor would not be treated as a violation; after an analytical result exceeds the MCL, the state
regulations call for follow-up sampling and analysis to determine whether a violation has occurred (New York State
Department of Health, Drinking Water Regulations, Part 5, Subpart 5-1, Public Water Systems – Tables).
Accordingly, the exceedances of regulatory thresholds would not be considered violations of New York State
regulations for public water supplies.
6
October were transitional for the system, which shifted between heating mode and cooling mode on
different days and different times of the same day. (On some spring and fall days, temperature data show
that the system was in cooling mode during the daytime hours when the school was occupied, but at night
it was in heating mode.)
During heating season, the lowest outflow water temperature measured at the time of sample collection
was 8.5° C, recorded in February 2016, and the largest temperature differential between inflow and
outflow samples was a reduction of 2.3 °C, recorded in December 2015. During cooling season, the
highest outflow water temperature measured at the time of sample collection was 21.3 °C, recorded in
September 2015, and the largest temperature differential was an increase of 4.3 °C, recorded on two
occasions in June and July 2016.
Comparison of temperatures measured at the time of sample collection with records of temperature at 15-
minute intervals from the heat-exchanger control system suggests that measurements at the time of
sample collection may not be representative of average conditions in the system, particularly during the
cooling season. Cooling-season outflow water temperatures and temperature differentials measured at the
time of sample collection (typically mid-to-late morning) are higher than the averages for that same full
day. For example, on 28 September 2016, the inflow and outflow water temperatures measured at the
time of sample collection were 15.0 and 17.8 °C, respectively, comparable to the values of 15.2 and 17.5
°C recorded at the same time by the control system, while 24-hour average temperatures for the same date
were 15.1 and 16.2 °C. Higher-than-average temperature differentials at the time of sample collection are
attributable to the time of day when sampling occurred, because daytime air temperatures usually exceed
the 24-hour average and because building occupancy at the time of sample collection contributes to the
cooling load.
There are no regulatory criteria for water temperature in a public water supply. Unusually high or low
temperatures might, however, affect customer satisfaction. Notably, in surveys and taste tests, Americans
have been found to express a preference for drinking water at cold temperatures; subjects in experiments
by Zellner et al. (1988) strongly preferred cold tap water at 0-5 °C over tap water served at room
temperature of 20-25 °C. Although water temperature can affect odor perception at temperatures above
the room temperature range (Whelton and Dietrich 2004) and can affect taste perception of soluble
substances such as sugar, salt, and acid, the preference for drinking cold tap water is attributed to
psychological factors, not to sensory characteristics of the water (Zellner et al. 1988, Green 1993). In
anticipation of returning heat exchanger outflow water to its Lynnbrook water distribution system,
NYAW has done a modeling evaluation of operational measures to ensure that the temperature of water
delivered to consumers would not increase by more than 3 °C as a result of passage through the heat
exchanger system (Jjemba 2018).
5.2.2 Biofilms
5.2.2.1 Context for biofilm measurements
The biofilm measurements were part of a larger suite of observations of bacterial water quality. The
investigations included measurements of three categories of bacteria:
1. Escherischia coli and total coliform bacteria. E. coli and total coliform bacteria are monitored and
regulated as indicators of possible contamination, particularly fecal contamination.
2. Heterotrophic bacteria, a broad category that includes all bact
eria that utilize organic carbon. The
heterotrophic bacteria detected in water testing are sometimes referred to as “heterotrophic plate
count bacteria” (HPC bacteria) to identify them as the subset of heterotrophic bacteria that are
isolated and cultured by the set of methods used for determining a “heterotrophic plate count.”
While certain heterotrophic bacteria can be pathogenic, HPC bacteria and HPC counts have not

7
been found to be relevant to human health risk (except possibly for severely
immunocompromised individuals) and there are no health-based standards for HPC bacteria in
drinking water (Allen et. 2004, Chowdhury 2012). Heterotrophic bacterial biofilms are of interest
in water distribution systems primarily because of concerns such as their potential role in pipe
corrosion, the possibility that biofilms on pipe walls could harbor pathogenic organisms, and
aesthetic concerns related to taste, odor, and discoloration of water or plumbing fixtures
(LeChevallier 1999
2
, Chowdhury 2012). The only U.S. or New York regulatory criterion for HPC
bacteria is a criterion intended to ensure that the presence of heterotrophic bacteria will not
interfere with detection of coliform bacteria.
3. Legionella, a bacterial genus that occurs in both natural waters and water distribution systems and
that has been associated with outbreaks of waterborne pathogenic disease.
All bi-weekly water samples were tested and found to be negative for both E. coli and total coliform
bacteria. All but 7 of the 78 heterotrophic plate counts (also known as “standard plate counts”) for bi-
weekly water samples found “less than” values of <1 or <2 for the “most probable number” of colony-
forming units per milliliter of water (MPN/mL); the other 7 samples returned values of 2 to 6 MPN/mL.
These counts are very low. For context, for public water systems supplied by surface water or
groundwater that is under surface water influence, New York regulations specify a heterotrophic plate
count result of 500 colonies per milliliter or less. This criterion is intended to ensure that the presence of
other heterotrophic bacteria will not interfere with detection of coliform bacteria (Allen et al. 2004).
Bacterial counts typically are very low in water supplies obtained from groundwater, such as the NYAW
Lynnbrook system.
5.2.2.2 Observations of biofilm formation
Biofilm measurements were obtained during the initial testing period in 2015-2016 and also from May
2017 (when the biofilm samples that were retrieved had been in the place for 8 months instead of the
typical 1 month) through October 2017. Results of biofilm measurements from the heat-exchanger water
lines are highly variable, probably due to factors such as the diversity of the organisms that could be
present. As shown in Table 1, the standard deviations for measurements of both ATP assay (ATP
accumulation rate) and plate count (HPC density) are larger than the mean values.
Results of measurements of biofilm formation were compared with values reported in other published
studies of bacterial biofilms in water systems. The values measured in the heat exchanger lines are low in
comparison with published values reported from other studies of bacterial biofilms, although it should be
noted that results of different studies may not be directly comparable because results may be affected by
methodological differences. A 15-month investigation of the effects of water characteristics on biofilm
formation in 26 U.S. drinking water supply systems (LeChevallier et al. 2015)
2
used an ATP assay
similar to the one in this study and measured biofilm formation rates that ranged from near zero to more
than 2.5 pg/mm
2
-d, with a mean value of 0.074 pg/mm
2
-d. The measurements from the heat-exchanger
study are in the low end of the ranges found in the published water supply study. The mean value reported
for all samples from inflow and outflow water lines for the Buck Elementary School heat-exchanger
system was an order of magnitude lower than the mean in the water supply study, at 0.0083 pg/mm
2
-d.
2
American Water company personnel were among the authors of this study.

8
Table 1. Summary statistics for all reported metrics of biofilm formation in inflow and outflow water lines
Inflow biofilm metrics Outflow biofilm metrics
ATP accumulation
rate (pg/mm
2
-d)
HPC density
(CFU/mm
2
)
ATP accumulation rate
(pg/mm
2
-d)
HPC density
(CFU/mm
2
)
Mean 0.010 882 0.006 968
Standard
deviation
0.026 1856 0.008 2032
In general, bacterial growth rates increase with increasing temperature, and the temperature 15°C has
been suggested as a threshold of interest for bacterial growth in water supplies. Studies of water supply
systems have found that the potential for occurrence of coliform bacteria is significantly higher when
water temperatures exceed 15 °C (LeChevallier 1999).
2
Also, the investigation of the effects of water
characteristics on biofilm formation in 26 U.S. water-supply systems identified 15 °C as a threshold water
temperature for biofilm formation (LeChevallier et al., 2015).
2
Below this temperature, rates of biofilm
formation were consistently very low (the study reported a mean ATP accumulation rate of 0.00426
pg/mm
2
-d for water less than 15 °C), but a substantial fraction of observations for water above this
temperature had significantly elevated rates of biofilm formation (the study reported a mean ATP
accumulation rate of 0.1035 pg/mm
2
-d for water above 15 °C). With this background, the biofilm data
from the heat exchanger study were examined for indications of a relationship between biofilm formation,
water temperature, and temperature changes resulting from the heat exchanger, including effects of
temperature above or below 15 °C.
For evaluation of whether increased water temperatures resulting to cooling-season operation of the heat
exchanger affected biofilm formation, the months of May through October were identified as
approximating the cooling season. Available water temperature data from these months indicate that for at
least part of the period when biofilms were forming inflow water temperatures were above 15 °C and the
temperature of water increased after passing through the heat exchanger system. Data from biofilm
samples that substantially formed during these months were examined for differences between inflow and
outflow values. Examination of the measurements of HPC density from biofilms from inflow and outflow
water lines (Table 2) suggests a possible trend toward higher values in the outflow lines during the
cooling season. Although the data in Table 2 suggest a tendency toward higher HPC densities in biofilms
formed in outflow water during the cooling season, because the data are highly variable and the
differences between outflow and inflow values are not statistically significant (differences between means
do not approach thresholds for statistical significance). Also, it is interesting to note that the highest
values of HPC density observed during the study were reported from samples collected from both inflow
and outflow lines in March 2016, when the system had been operating in heating mode and water
temperatures were well below 15 °C.
Measurements by ATP assay, which are based on biochemical indicators of bacterial activity, may have
greater reproducibility than measurements based on counting bacterial colonies. Duda et al. (2015)
reported good correlation between HPC plate counts and ATP assays on water samples. They observed
that ATP assays of water samples showed less variability than HPC plate counts. As with HPC density,
examination of biofilm accumulation rates for inflow and outflow biofilm samples formed during the
cooling season suggests a possible tendency toward higher values in outflow water lines (Table 3), but the
differences are not statistically significant (differences between means do not approach thresholds for
statistical significance).

9
Table 2. HPC bacterial densities determined from biofilms formed during the cooling season
Biofilm Collection Date
HPC density (HPC/mm
2
)
Inflow Line Outflow Line
3 September 2015
17 30
24 55
8 October 2015
0 0
1 118
9 November 2015
2 26
4 125
15 June 2016
209 242
314 1864
14 July 2016
413 365
503 581
24 August 2016
1232 1616
1357 1776
28 September 2016
240 121
435 412
22 June 2017
30 200
41 310
31 July 2017
290 71
770 690
30 August 2017
38 140
1300 770
10 October 2017
480 350
660 830
Mean
380 486
Standard Deviation
428 557
Note: Two coupons were placed in each water line during each sampling period.
HPC densities determined for the individual biofilm samples are reported
separately. HPC bacterial densities were not determined for biofilm samples
collected on 6 Au
g
ust 2015.
To assess whether biofilm accumulation rates in this study were increased by higher water temperature,
all accumulation rates determined from ATP assays on samples collected in 2015 and 2016 were plotted
against the average temperature in the time period when the biofilm was forming, as shown in Figure 1
(temperatures in this graph were determined as the mean of the temperatures at the times when water
samples were collected). Figure 1 illustrates that there is no observable relationship between biofilm
formation rate and temperature. It is interesting to note that the highest observed ATP accumulation rate
was from a biofilm (collected in December 2015) that formed in an inflow water line during a period
when water temperature was below 15 °C.
10
Table 3. ATP accumulation rates determined from biofilms formed during the cooling season
Biofilm Collection Date
ATP accumulation rate (pg/mm
2
-d)
Inflow Line Outflow Line
6 August 2015
0.0005 0.0017
0.0045 0.0071
3 September 2015
0.0004 0.0003
0.0006 0.0004
8 October 2015
0.0048 0.0048
0.0050 0.0110
9 November 2015
0.0014 0.0001
0.0016 0.0002
15 June 2016
0.00285 0.00035
0.00311 0.02705
14 July 2016
0.00384 0.00408
0.00441 0.00762
24 August 2016
0.00091 0.00055
0.00186 0.00629
28 September 2016
0.00374 0.00333
0.00514 0.00570
22 June 2017
0.003 0.003
0.005 0.005
31 July 2017
0.007 0.002
0.007 0.015
30 August 2017
0.008 0.004
0.029 0.019
10 October 2017
0.009 0.004
0.015 0.015
Mean 0.0053
.0061
Standard Deviation 0.0059
.0067
Note: Two coupons were placed in each water line during each sampling period.
HPC densities determined for the individual biofilm samples are reported
separatel
y
.

11
Figure 1. ATP accumulation rates (mean of two samples) for biofilms vs. mean temperature recorded in
sampling events in 2015 and 2016 during the period when the biofilm was forming.
5.2.2.3 Investigations of Legionella
Legionella was detected in one biofilm sample during the initial testing period in 2015 and 2016. The
sample was from a biofilm collected from the outflow line on 6 August 2015. Legionella is widely
distributed in waters, typically at very low levels, and has been found in biofilms in water distribution
systems. Filtration and disinfection of water supplies do not prevent the growth of Legionella, but this
organism grows slowly and is difficult to culture. The significance of isolated observations of Legionella
is uncertain. Disease outbreaks result from inhalation of Legionella in water droplets, and have been
associated with cooling towers, hot tubs, shower heads, and recirculating hot water systems. Studies have
found that Legionella does not proliferate except in the presence of protozoa, particularly amoebae
(Committee on Public Water Supply Distribution Systems 2006).
To determine whether Legionella is present in the system and evaluate the significance of the finding,
NYAW conducted additional coupon testing on five sets of coupon samples collected between May and
October 2017 (a total of twenty samples, including two from the inflow line and two from the outflow
line, collected on each of five dates). Collection and testing of biofilm samples in 2017 followed the
same methodologies used for samples collected in 2015-2016, with the addition of quantitative
polymerase chain reaction (qPCR) methodology to evaluate the presence of Legionella bacteria.
Quantitative PCR has been demonstrated to be more effective at detecting Legionella than cell culture,
thus reducing the potential for false negative results, and it enables quantification of the presence of this
microorganism (Whiley and Taylor 2016; Collins et al. 2017). This methodology does, however,
introduce a potential for false positives, as it does not distinguish whether detected DNA is from viable
living Legionella or from dead or nonviable organisms (Whiley and Taylor 2016). Detection of
Legionella in environmental samples using qPCR has been described as a widely accepted method that is
growing in popularity (Collins et al. 2017).
The methodology for the Legionella investigation is described in reports provided to ORNL by NYAW
(Jjemba 2018; Morris 2018; see Appendix B). DNA was extracted from a biofilm sample using a
commercially available kit, eluted with water to a final volume of 50 uL, and amplified using rRNA
specific primers targeting the variable 23S-5S ribosomal intergenic spacer region of Legionella, applying
a technique described by Grattard et al. (2006). Quantification was conducted against a standard curve
established from analysis of ten-fold dilutions of L. pneumophila strain Philadelphia 1, using methods
based on published literature. Genetic sequences were evaluated against a National Center for
Biotechnology Information database to determine their identity, including differentiating the species L.
12
pneumophila. The great majority of Legionella infections are attributed to species L. pneumophila,
although other species of Legionella are also capable of causing human infection, particularly in
immunocompromised individuals (Grattard et al. 2006, Yang et al. 2010). Because the qPCR
methodology used by NYAW followed published methodologies, and the same methods were used for
biofilm samples from inflow and outflow water lines, the analyses are judged to be suitable as a basis for
comparative evaluation of Legionella presence in inflow and outflow water lines.
Legionella was not found in cultures from any of the samples obtained in 2017, but DNA markers for
Legionella were detected in nine of ten samples from inflow lines (at least one sample from every
sampling date) and five of ten samples from outflow lines (at least one sample from four of the five
sampling dates). For both inflow and outflow lines, quantification indicated the presence of 40 to 119
gene copies per mm
2
coupon surface in samples that had Legionella markers. Sequencing of material
from these fourteen samples confirmed the presence of Legionella in three samples and provided
uncertain results for three samples; sequencing failed to identify Legionella in the remaining samples.
Five inflow samples collected on four dates (May, July, August, and October) had identified or uncertain
presence of Legionella and one outflow sample collected in June had identified or uncertain presence of
Legionella. L. pneumophila, the species implicated in the large majority of human disease cases, was
identified in just one of the positive samples (one inflow sample collected in October, in a sample that had
40 gene copies per mm
2
); the other five identifications were of undetermined species of Legionella.
The detection of markers for Legionella in a large fraction of samples is generally consistent with the
findings of other studies that have used qPCR to test water samples for this organism and have found it in
a large fraction of various types of natural waters and water supplies that are tested (Whiley and Taylor
2016; Collins et al. 2017). While Legionella is a cause for public-health concern, detection of this
ubiquitous organism at low levels can be interpreted as indicating only low risk (Whiley and Taylor
2016). Its presence in both inflow and outflow biofilm samples from the heat exchanger study, including
its presence in more inflow samples than outflow samples, supports a conclusion that passage of water
through the heat exchanger is not promoting the occurrence of
Legionella in this system.
6. SUMMARY CONCLUSIONS
In summary, water quality data supplied by New York American Water were found to be suitable for the
purposes of the evaluation. Data were compared with regulatory standards and inflow and outflow data
were compared to identify changes occurring in the water as a result of its passage through the heat
exchanger. Review of the data identified no conditions that would prevent the use of heat exchange
outflow water for water supply. Specifically:
1. Water quality conforms with applicable regulatory standards.
2. There are no discernible differences between inflow and outflow water quality for any parameters
other than water temperature and growth of heterotrophic bacteria in biofilms. The observed
differences in bacterial growth in biofilms are inconsistent, and differences between inflow and
outflow are not statistically significant.
3. Changes in water temperature are an expected result of the operation of the heat exchanger
system and do not by themselves affect the suitability of the water for human consumption.
4. Bacterial biofilm formation can be affected by water temperature, but the data did not show a
consistent or statistically significant relationship between temperature parameters and biofilm
formation, and all measured values of biofilm formation were very low. Heterotrophic bacterial
biofilms are common in water distribution systems. They are not normally a source of concern for
human health and are not subject to regulatory standards. Accordingly, the observations of
13
biofilms formed in the heat exchanger water lines do not affect the suitability of the outflow water
for water supply.
5. Legionella, a bacterial genus that is found in many waters and that has been associated with
outbreaks of waterborne pathogenic disease, was detected in biofilms retrieved from both inflow
and outflow water lines. Its presence in both inflow and outflow biofilm samples from the heat
exchanger study supports a conclusion that passage of water through the heat exchanger is not
promoting the occurrence of
Legionella in this system. Moreover, although the potential presence
of Legionella in a water supply cannot be dismissed as a public health concern, this organism has
been detected in many water sources and water supplies, so its detection at low levels in this
investigation does not indicate any particular risk.

14
7. REFERENCES
Allen, Martin J., Stephen C. Edberg, and Donald J. Reasoner. 2004. Heterotrophic plate count bacteria –
What is their significance in drinking water? International Journal of Food Microbiology 2004 92,
265-274.
Chowdhury, Shakhawat. 2012. Heterotrophic bacteria in water distribution system: a review. Environ.
Monit. Assess. 184, 6087-6137. DOI 10.1007/s10661-011-2407-x
Collins, S., D. Stevenson, J. Walker, and A. Bennett. 2017. Evaluation of Legionella real-time PCR
against traditional culture for routine and public health testing of water samples. Journal of Applied
Microbiology 122:1692-1703. DOI 10.1111/jam.13461
Committee on Public Water Supply Distribution Systems. 2006. Drinking Water Distribution Systems:
Assessing and Reducing Risks. National Academies Press, Washington, DC.
http://www.nap.edu/catalog/11728.html
Duda, Scott, Julianne L. Baron, Marilyn M. Wagner, Radislav D. Vidic, and Janet E. Stout. 2015. Lack of
correlation between Legionella colonization and microbial population quantification using
heterotrophic plate count and adenosine triphosphate bioluminescence measurement. Environ. Monit.
Assess. 187:393. DOI 10.1007/s10661-015-4612-5.
Department of Energy (DOE). 2016. Geothermal Heat Pumps;
https://energy.gov/energysaver/geothermal-heat-pumps.
Grattard, Florence, Christophe Ginevra, Serge Riffard, Alain Ros, Sophie Jarraud, Jerome Etienne, and
Bruno Pozzetto. 2006. Analysis of the genetic diversity of Legionella by sequencing the 23S-5S
ribosomal intergenic spacer region: from phylogeny to direct identification of isolates at the species
level from clinical specimens. Microbes and Infection 8: 73-83. DOI: 10.1016/j.micinf.2005.05.022
Green, Barry G. 1993. Heat as a Factor in the Perception of Taste, Smell, and Oral Sensation. pp. 173-185
in: Nutritional Needs in Hot Environments: Applications for Military Personnel in Field Operations,
Bernadette M. Marriott, ed. Washington, DC: National Academy Press.
http://www.nap.edu/catalog/2094.html
Jjemba, Patrick K. 2018. Additional Evaluation of the Impacts of Heat Exchanger Operation on
Distribution Water Quality. American Water Research Laboratory, Delran, NJ. February 13, 2018.
LeChevallier, Mark W. 1999. Biofilms in Drinking Water Systems: Significance and Control. Chapter 10.
In Identifying Drinking Water Contaminants. National Academy Press, Washington, DC.
https://www.nap.edu/read/9595/chapter/12.
LeChevallier, Mark W., Orren D. Schneider, Lauren A. Weinrich, Patrick K. Jjemba, Patrick J. Evans,
Jennifer L. Hooper, and Rick W. Chappell. 2015. An Operational Definition of Biostability in
Drinking Water. Water Research Foundation, Denver, CO. Downloaded from
http://www.waterrf.org/PublicReportLibrary/4312b.pdf.

15
Lombardo, Tom. 2015. Innovative Approach to Geothermal Heating and Cooling. Engineering.com.
October 19, 2015.
http://www.engineering.com/DesignerEdge/DesignerEdgeArticles/ArticleID/10830/Innovative-
Approach-to-Geothermal-Heating-and-Cooling.aspx.
Morris, Kendra F., American Water. 2018. Email communication to Ellen D. Smith, ORNL, February 19,
2018.
New York American Water. 2016. 2015 Annual Water Quality Report, Lynnbrook Operations District.
New York American Water. undated. NYAWC Geothermal Project Laboratory Materials and Methods.
Sent to ORNL via email by Patrick K. Jjemba, January 21, 2017.
New York State Department of Health, Drinking Water Regulations, Part 5, Subpart 5-1, Public Water
Systems – Tables, revised November 2011. Accessed at
https://www.health.ny.gov/regulations/nycrr/title_10/part_5/subpart_5-1_tables.htm
Whelton, Andrew J., and Andrea M. Dietrich. 2004. Relationship between intensity, concentration, and
temperature for drinking water odorants. Water Research 38: 1604-1614.
Whiley, Harriet, and Michael Taylor. 2016. Legionella detection by culture and qPCR: Comparing apples
and oranges. Critical Reviews in Microbiology, 42:1, 65-74. DOI:10.3109/1040841S.2014.885930
Yang, G., R. Benson, T. Pelish, E. Brown, J. M. Winchell, and B. Fields. 2010. Dual detection of
Legionella pneumophila and Legionella species by real-time PCR targeting the 23S-5S rRNA gene
spacer region. 2009. Clinical Microbiology and Infection 16: 255-261. DOI: 10.1111/j.1469-
0691.2009.02766.x
Zellner, D. A., W. F. Stewart, P. Rozin, and J. M. Brown. 1988. Effect of temperature on expectations and
liking for beverages. Physiology & Behavior 44: 61-68.
APPENDIX A. SUMMARY LIST OF ANALYTES REPORTED TO
ORNL BY NYAW

A-3
SUMMARY LIST OF ANALYTES REPORTED TO ORNL BY NYAW
Water Analyses
Inorganic Analytes
Calcium
Chloride
Fluoride
Iron
Magnesium
Manganese
Sodium
Sulfate
Zinc
Antimony
Arsenic
Barium
Beryllium
Cadmium
Chromium
Copper
Lead
Mercury
Nickel
Selenium
Silver
Thallium
Alkalinity, Total (as CaCO
3
)
Hardness, Calcium (as CaCO
3
)
Total Hardness (as CaCO
3
)
Total Dissolved Solids
Ammonia (as N)
Nitrate (as N)
Purgeable Volatile Organics
1,1,1,2-Tetrachloroethane
1,1,1-Trichloroethane
1,1,2,2-Tetrachloroethane
1,1,2-Trichloroethane
1,1-Dichloroethane
1,1-Dichloroethene
1,1-Dichloropropene
1,2,3-Trichlorobenzene
1,2,3-Trichloropropane
1,2,4-Trichlorobenzene
1,2,4-Trimethylbenzene
1,2-Dichlorobenzene
1,2-Dichloroethane
1,2-Dichloropropane
1,3,5-Trimethylbenzene
1,3-Dichlorobenzene
1,3-Dichloropropane
1,4-Dichlorobenzene
2,2-Dichloropropane
2/4-Chlorotoluene
4-Isopropyltoluene
Benzene
Bromobenzene
Bromochloromethane
Bromodichloromethane
Bromoform
Bromomethane
Carbon tetrachloride
Chlorobenzene
Chloroethane
Chloroform
Chloromethane
cis-1,2-Dichloroethene
cis-1,3-Dichloropropene
Dibromochloromethane
Dibromomethane
Dichlorodifluoromethane
Ethylbenzene
Hexachlorobutadiene
Isopropylbenzene
m,p-Xylene
Methyl tert-butyl ether
Methylene chloride
n-Butylbenzene
n-Propylbenzene
o-Xylene
sec-Butylbenzene
Styrene
tert-Butylbenzene
Tetrachloroethene
Toluene
Total Trihalomethanes (calculated as sum of individual
trihalomethanes)
trans-1,2-Dichloroethene
trans-1,3-Dichloropropene
Trichloroethene
Trichlorofluoromethane
Vin
y
l chloride

A-4
Other Water Analysis Parameters
Free Chlorine Residual (field)
Free Cyanide
Temperature (field)
pH (field)
E. coli
Total coliform
Heterotrophic Plate Count
Color
Odor at 60 °C
Methylene Blue Active Substances (MBAS)
Turbidity
Langelier saturation index (LSI; calculated
from other parameters)
Corrosion Coupon Analyses
Corrosion Rate
Pit Index
Le
g
ionella culture and qPCR data
ATP Accumulation Rate
Heterotrophic Plate Count (CFU/mm
2
)
APPENDIX B. METHODOLOGY DESCRIPTIONS PROVIDED
TO ORNL BY NYAW
B-3
NYAWC Geothermal Project Laboratory Materials and Methods
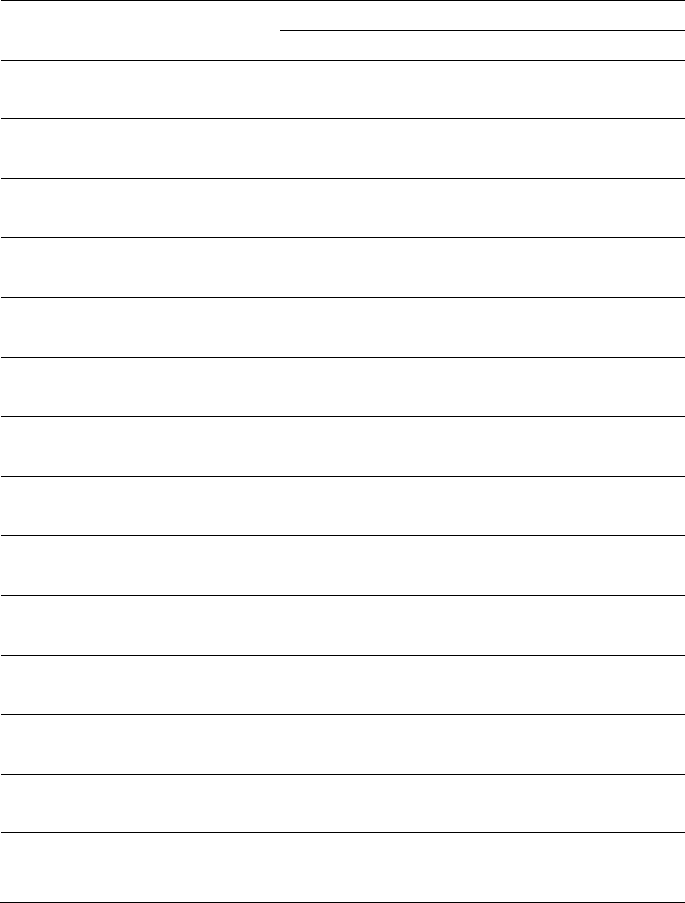
Metal coupon system setup
Mild steel coupons (P/N CO100375104100, dimensions of 3” x ½” x 1/16”; 1-Hole Strip)
were purchased from Alabama Specialty Products, Inc., Munford, AL. inserting metal
coupons at the entrance and terminal of the heating/cooling system. For duplication, two
coupons (A and B) were inserted into the flow stream using a retractable holder (ASPI,
2015; Figure 1). A similar setup was established at the heat exchanger effluent side. The
retractable holder enabled aseptically removing the coupon from the pressurized pipe
without shutting down the flow. Each
coupon was be left in place for one month
and exposed to a continuous flow to
develop a biofilm. A new coupon was
replaced each month as to compare
relative growth rates throughout the year.
Thus, a total 48 coupons (i.
e., 2
locations/month × 2 duplicates × 12
months of sampling) were analyzed. At
the appropriate time, each coupon was
removed and placed in a sterile 50-mL
centrifuge tube and the tube filled with
indigenous water to avoid biofilm
shearing or drying in transit. The tubes
were shipped overnight on ice (4
o
C to 8
o
C) the American Water laboratory in
Delran for analysis.
Biofilm recovery and analysis
In the laboratory, the residual water was decanted
from the tube and the coupon will be submerged in
20 mL sterile phosphate buffer (3 mg KH
2
PO
4
/L
and 7 mg K
2
HPO
4
/L; pH 7). The biofilm was
carefully scrapped off the metal coupon with a
sterile brush and each tube filled with phosphate
buffer up to the 30-mL mark (Figure 2) to provide
a uniform biofilm suspension. ATP assay uses a
single reagent (BacTiter-Glo
TM
Reagent) added to
a biofilm suspension and measuring the
luminescence. The BacTiter-Glo
TM
Reagent relies
on the properties of a proprietary thermostable
luciferase enzyme isolated from fireflies. The
enzyme requires energy from ATP to produce light and the kit also has a proprietary
formulation for extracting ATP from microorganisms. Triplicate aliquots of 100 μL biofilm
Figure1.Couponassemblyandwaterflow
Water out
Water in
Coupon
A
Coupon
B
Connection to
corrosion meter
Figure2.Typicalcoupon‐derivedbiofilmsuspensionused
forATPdetermination.

B-4
suspension was added to an equal volume of BacTiter-Glo™ Reagent (Promega, Madison,
WI). The mixture was incubated at 30 °C for 1.5 min with periodic mixing every 30
seconds. Luminescence (relative light units) was measured exactly 30 s using the
GloMax
TM
20/20 luminometer (Promega, Madison, WI). The luminescence value was
converted to ATP concentrations based on a calibration curve obtained by spiking serial
dilutions of a 10mM ATP stock incubated with the biofilm suspension which had been
inactivated by heating at 60
o
C (21 hours) and then treated as any other sample (Veltens et
al., 2007; LeChevallier et al., 2015). A typical calibration curve is shown in Figure 3. ATP
results were ultimately expressed on a pg ATP/cm
2
basis as summarized in a sample set of
raw data in Table 1.
Figure 3. A typical standard calibration curve with an ATP stock serial diluted by spiking
in deactivated a biofilm suspension.
An aliquot the biofilm suspension was used to determine heterotrophic bacteria in the
biofilm using the spread-plate method (Standard Method 9215C [Eaton et al., 2005]) on
R2A agar. The plates were incubated at 22±1
o
C for one week. Heterotrophic bacteria
(typically referred to as HPCs) are used frequently in the water industry to provide
information about the microbiological and aesthetic quality of drinking water. The results
were expressed on a per unit area (i.e., HPC/mm
2
) of the coupon.
y=0.888x+8.7749
R²=0.9918
0
1
2
3
4
5
6
7
8
9
10
‐8 ‐7 ‐6 ‐5 ‐4 ‐3 ‐2 ‐1 0
Logluminscence(RLU)
LogATPconcentration

B-5
Table 1. Sample spreadsheet entry used to calculate the ATP accumulation rate in the coupon biofilm
Coupon
ID Rep
Luminescence
(RLUs)
Log
luminescence
Log ATP
(ug/100uL) ATP (ug/100uL)
ATP
(ug/30mL)
ATP
(ug/mm
2
)
ATP
(pg/mm
2
)
Installation
date
Harvest
date
Days
in DS
Accumulation ate
(pgATP/mm2/day)
INF‐A‐
04202016
1 5389 3.731508184
‐5.679495289 2.09173E‐06 0.000627518 4.27464E‐07 0.427464359 3/16/2016 4/20/2016 35 0.012213267
2 5046 3.702947246 ‐5.711658507 1.94241E‐06 0.000582724 3.96951E‐07 0.39695081 3/16/2016 4/20/2016 35 0.011341452
3 5277 3.722387094 ‐5.689766786 2.04283E‐06 0.00061285 4.17473E‐07 0.41747302 3/16/2016 4/20/2016 35 0.011927801
Mean 5237.333333 2.02566E-06 4.13963E-07 0.41396273 0.011827507
SD 174.9066418 7.61241E-08 1.55567E-08 0.015556695 0.000444477

B-6
Duplicate 100 µL aliquots of the biofilm suspension were plated on BCYE agar
supplemented with GVPC (Oxoid) and a Legionella agar enrichment (BD Difco, Sparks,
MD). To ensure detection of low levels of Legionella, another aliquot of 20 mL biofilm
suspension was filtered through a 0.2 µm 47-mm diameter polycarbonate filter. The filter
was aseptically removed and inserted into a tube containing 5 mL sterile water. The tube
was vortexed at high speed to re-suspend the bacteria. To eliminate non-Legionella
organisms during this selection growth process, 1 mL of the sample was pretreated with
acidified potassium chloride (0.2 M KCl/HCl; pH = 2.2) for 15 minutes at room
temperature and thereafter 0.1 mL spread-plated on BCYE agar supplemented with GVPC
(Oxoid) and a Legionella agar enrichment (BD Difco, Sparks, MD). Thereafter, an aliquot
of 0.1 mL was streaked on BCYE agar supplemented with GVPC (Oxoid) and a Legionella
agar enrichment (BD Difco, Sparks, MD). This extra step is recommended under CDC
guidelines for detecting low levels of Legionella in environmental samples. The plates were
incubated at 36.5°C with 2.5% CO
2
and 94% relative humidity. Growth on the plates was
monitored for up to 10 days. Though any growth was ever detected, where it occurred, the
presumptive Legionella sp. colonies were streaked on BCYE without any cysteine. Most
species require iron salts and cysteine for growth (Eaton et al., 2005; Lück et al., 2004).
Failure to grow in the absence of cysteine confirmed the streaked parent colonies as
Legionella sp. (NHS, 2007).
References
ASPI (2015) Length calculation and accessories for retractable probes and coupon
holders (URL: http://www.alspi.com/length.pdf; accessed 2/13/2015)
Eaton, A. D., Clesceri, L.S., Rice, E.W., Greenberg, A.E., Eds. Standard Methods for the
Examination of Water and Wastewater, 21st ed.; American Public Health Association:
Washington, DC, 2005.
LeChevallier M.W., O.D. Schneider, L.A. Weinrich, P.K. Jjemba, P.J. Evans, J.L.
Hooper, and R.W. Chappell (2015b) An Operational Definition of Biostability in
Drinking Water. Water Research Foundation Report# 4312b, Denver, Colorado.
(http://www.waterrf.org/PublicReportLibrary/4312b.pdf).
Lück, P. C.; Igel, L.; Helbig, J. H.; Kuhlisch, E.; Jatzwauk, L. Comparison of
commercially available media for the recovery of Legionella species. Int. J. Hyg.
Environ. Health 2004, 207, 589–593.
NHS. Identification of Legionella Species. http://www.hpa-
standardmethods.org.uk/documents/bsopid/pdf/bsopid18.pdf (accessed Nov 2007), 2007.
Promega (2009) Technical Bulletin – BacTiter-Glo
TM
Microbial Cell Viability Assay.
Part TB337.
Velten S., F. Hammes, M. Boller, and T. Egli (2007) ATP measurement as a means for
directly estimating active biomass. Promega Notes 97:15-17.
B-7
Additional Evaluation of the Impacts of Heat Exchanger Operation on
Distribution Water Quality
Patrick K. Jjemba PhD
American Water Research Laboratory, Delran NJ
February 13, 2018
Summary
This report describes the results of the second water quality test performed on municipal water
running through a geothermal heat exchanger. The test’s objective was to determine whether the
heat exchanger would cause an increase in Legionella levels in the water passing through the
system. American Water’s Water Research and Development department used a culture-based
method and a PCR method to identify any change in Legionella and based on the evidence
collected to date, has concluded the heat exchanger does not appear to increase Legionella risk in
the effluent water.
Using strict protocols for testing for the presence of Legionella on the influent and effluent
coupons, we found zero culturable Legionella in any sample, but found DNA markers for
Legionella that were confirmed as originating from Legionella in only 10% of the samples (six
out of 60 total). Of the six samples, Legionella was detected in the influent in four different
months and only once in the effluent in a separate month. The results demonstrated that within
this testing period the heat exchange system did not result in an increase in Legionella, even with
an observed average temperature increase of 2.9°C.
Additional modeling work was completed to simulate the impact of returning the higher
temperature effluent water to the drinking water distribution system. While several options were
modeled under highly conservative conditions (i.e., assuming that temperature was conserved and
only impacted by dilution, but not dissipating heat to the piping or surrounding earth), two
scenarios were able to keep any impact to the local distribution system to less than 3 °C increase
anywhere within the influence area.
Background
A geothermal heat pump system was installed on a potable water distribution main in 2015 to
provide heating and cooling to a 40,000 square foot elementary school in New York. Water
passed through the heat exchanger is currently running to waste pending a permit from the local
Department of Health (DOH) to reinject it into the distribution main and “close the loop.”
American Water performed water quality testing during the cooling season to understand the heat
exchanger’s impact to water quality as a result of transferring heat from the building to the
municipal water. This timeframe was selected as the system is intensively used at that time (i.e.,
cooling season) which can increase water temperature resulting in Legionella growth. The first
testing occurred during two consecutive cooling seasons and the second testing occurred during
the following cooling season (Table 1).

B-8
Table 1. Testing Phases and Dates
Testing Phase Dates
1 August 2015 -September 2016
2 May – October 2017
Legionella detection during the first testing phases was solely based on a laboratory growth
medium (i.e., BCYE agar) and showed presumptive Legionella in one out of 24 coupons (i.e.,
4.2%) tested. The presumptive Legionella were at a very low concentration of 1.2 colony forming
units (cfu) per 4mm
2
of the coupon surface area. However, genotype confirmation of the presence
of Legionella was not possible due to loss of the sample prior to re-analysis.
Out of an abundance of caution, monitoring was extended through the following spring and
summer period (i.e., May to October 2017). The second testing phase mimicked the first testing
phase’s use of the conventional culture method and added a more sensitive molecular analysis
technique (qPCR). In the second testing phase, we specifically wanted to determine whether the
heat exchanger increases the risk of Legionella amplifying in the outflow line.
Methodology and Modeling Information Provided in the Appendix
Results and Discussion
Water pH, chlorine residual and temperature during the testing period is summarized in Table 2
and are shown graphically in Figure 1 and 2. As expected, temperature increases across the heat
exchange system while the chlorine residual was maintained, indicating stability of the
disinfectant through the system. Water pH was mostly unchanged except in mid-July when pH
drops (i.e., increase in acidity) of 0.2 to 1 unit were recorded. The pH drop was significantly
different from maximum pH in the influent and effluent. However, these observed changes in pH
are more likely due to sensor drift between the influent and effluent lines than any real change in
pH due to the lack of chemical mechanism, which would explain such a shift.
Table 2: Summary of physicochemical water quality throughout testing phase 2
Statistical
parameter
Water pH Chlorine residual (mg/L) Water temperature (
o
C)
Influent Effluent Difference Influent Effluent Difference Influent Effluent Difference
Minimum 5.33 5.16 -0.17 0.3 0.3 0 -0.25 1.96 2.21
Maximum 7.24 7.17 -0.07 1.11 1.14 0.03 16.86 19.62 2.76
Mean 6.25 6.02 -0.23 0.66 0.67 0.01 10.76 13.67 2.91
Standard deviation 0.56 0.49 0.27 0.28 4.32 3.19
Lsd (p<0.05)
1
1.31 1.41 (NS)
2
1.31
1
Lsd = least significant difference at the 5% level of significance
2
NS = Not significantly different
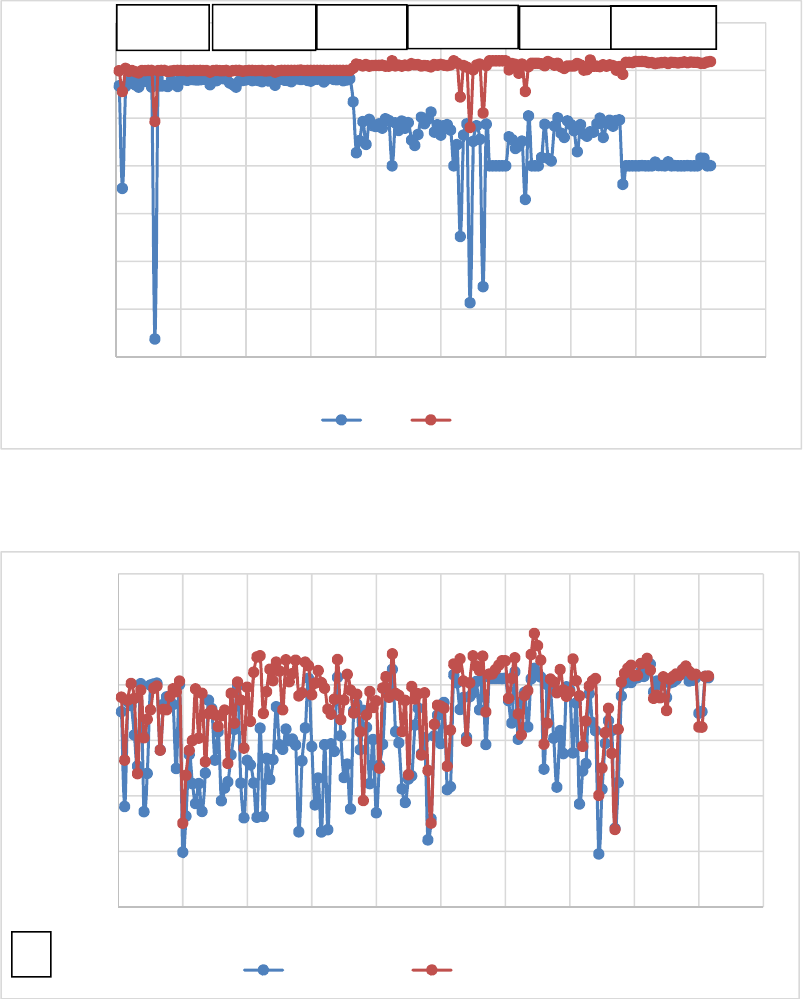
B-9
Figure 1. Difference (∆) in (A) water pH and chlorine residual
(1.20)
(1.00)
(0.80)
(0.60)
(0.40)
(0.20)
0.00
0.20
0 20 40 60 80 100 120 140 160 180 200
ΔpHandΔFreeChlorine(mg/L)
ΔpH ΔCl2
(5.00)
0.00
5.00
10.00
15.00
20.00
25.00
0 20 40 60 80 100 120 140 160 180 200
Temperature(
o
C)
PreTemp(oC) PostTemp(oC)
May
OctSept
Aug
July
June
A

B-10
Figure 2. Temperature trends (A) in the pre- and post heat exchanger (B) corresponding
differences (∆) in the effluent compared to the influent
The ATP accumulation rate, indicating the level of microbial activity, was not different in the
influent and effluent to the heat exchanger. Mean biofilm accumulation rates were 0.009 pg
ATP/mm
2
/day and 0.010 pg ATP/mm
2
/day in the influent and effluent loops respectively. The
low biofilm accumulation rate is probably indicative of the stable groundwater used in this study.
By comparison, a survey of 26 U.S. drinking water supply systems had mean ATP accumulations
rates seven times as high (i.e., 0.074 pg ATP/mm
2
/day; LeChevallier et al., 2015).
Heterotrophic bacteria (HPC) concentrations varied from month to month. We calculated the
geometric mean to “normalize” the heterotrophic bacteria densities and found that the mean
influent and effluent HPCs were not statistically different after factoring in the standard deviation.
Thus, the biofilm did not significantly change as water flowed through the heat exchanger
indicating the geothermal system did not negatively impact water quality. Likewise, the
relationship between ATP and HPC were identical in pre- and post-exchanger as show in Figure
3. The lack of change in relationship between ATP and HPC indicates that there was no change
in the water quality that would favor bacterial regrowth in the water.
Table 3 provides an overview of the various water quality parameters that were measured to
analyze the heat exchanger’s impact on water quality on the days the coupons were retrieved.
(10.00)
(5.00)
0.00
5.00
10.00
15.00
20.00
0 20 40 60 80 100 120 140 160 180 200
ΔTemperature(
o
C)
B

B-11
Table 3: Geothermal unit influent and effluent attributes on days the coupons were retrieved
Influent
Effluent
Sampling date Coupon
ATP
accumulation
rate
(pg/mm
2
/day)
Legionella
/mm
2
HPC
(CFU/mm2)
Temp
(
o
C)
Cl
(mg/L)
pH
ATP
accumulation
rate
(pg/mm
2
/day)
Legionella
/mm
2
HPC
(CFU/mm
2
)
Temp
(
o
C)
Cl
(mg/L)
pH
CFU
¶
Gene
copies
CFU
¶
Gene
copies
May, 19 2017
◊
A 0.007 0 119 870 15.0 0.62 7.12 0.035 0 0 2100 15.3 0.62 7.05
B 0.002 0 79 300
0.002 0 119 350
June, 22 2017 A 0.003 0 40 41 10.0 0.4 6.06 0.003 0 79 200 15.3 0.4 6.02
B 0.005 0 119 30
0.005 0 40 310
July, 31 2017 A 0.007 0 79 290 11.4 0.87 6.39 0.002 0 0 71 13.7 0.89 6.07
B 0.007 0 40 770
0.015 0 119 690
August, 30 2017 A 0.029 0 79 38 11.6 0.8 6.2 0.004 0 40 140 15.6 0.83 5.91
B 0.008 0 40 1300
0.019 0 0 770
October, 10 2017 A 0.009 0 40 480 15.8 1.1 7.04 0.004 0 0 350 17.0 1.14 6.64
B 0.015 0 0 660
0.015 0 0 830
Mean
0.009 0 63.5 250* 12.7 0.76 6.56 0.010 0 39.7 320* 15.4 0.78 6.28
SD
0.008 0 38.2 420 2.5 0.26 0.49 0.010 0 49.4 280 1.2 0.28 0.52
¶
CFU=Colony forming units (i.e., number of microbial colonies formed on laboratory growth media in culture testing method as an indicator of Legionella
colony abundance)
◊
The coupons harvested in May were in place for 8 months as compared to all of the others which were in place for about one month
*Geometric mean

B-12
Figure 3. Relationship between HPC and ATP in pre- and post-heat exchanger biofilms showing same-slope lines to demonstrate central tendency
of data.
0.000
0.005
0.010
0.015
0.020
0.025
0.030
0.035
0.040
0 500 1000 1500 2000 2500 3000 3500
ATP(pg/mm
2
/day)
InfluentHPC/mm
2
0.000
0.005
0.010
0.015
0.020
0.025
0.030
0.035
0.040
0 500 1000 1500 2000 2500 3000 3500
ATP(pg/mm
2
/day)
EffluentHPC/mm
2
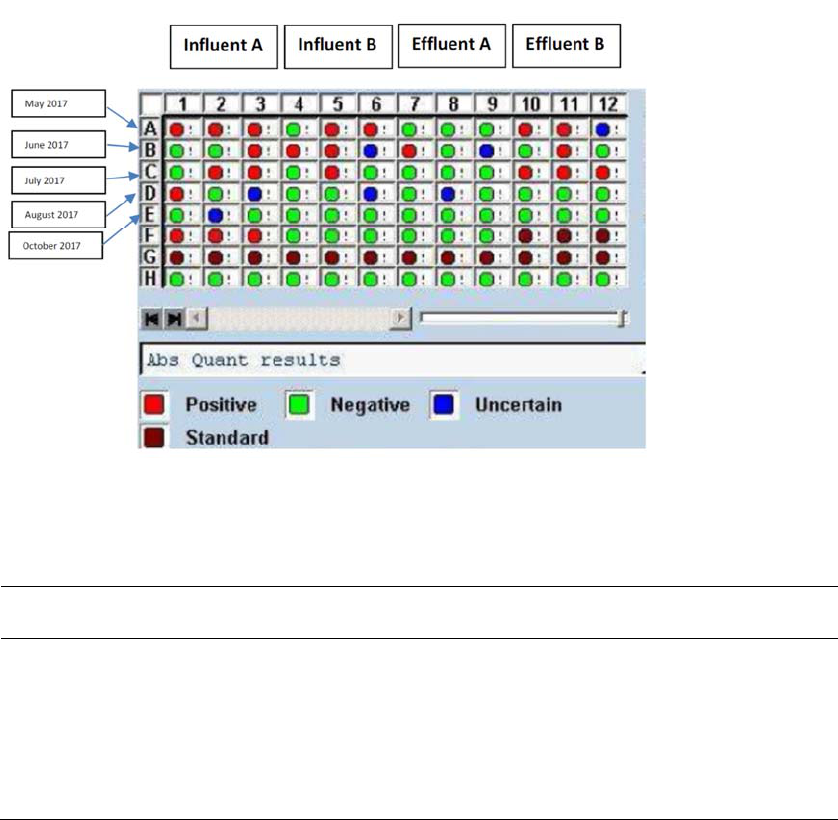
B-13
PCR and DNA Sequencing
While the culture method did not detect Legionella, five influent and one effluent samples out of 60 were
positive for Legionella using the qPCR molecular method and sequencing (Figure 4). The PCR test
assigns each sample a positive, uncertain or negative indicator expressed through red, blue and green dots.
A positive indicator (red dot) means the PCR instrument identified an organism with certain predefined
DNA targets. An uncertain indicator (blue dot) means the PCR instrument is uncertain if the organism
matches the predefined DNA targets. In the initial PCR run, as seen in rows A – E in Figure 3, there were
10 positive and uncertain PCR wells for the effluent samples compared to 16 positive and uncertain wells
in the influent.
In order to determine if the PCR results were in fact Legionella and to identify the species of Legionella,
additional DNA sequencing was conducted on the positive and uncertain samples. On sequencing, only
three of PCR products were confirmed as Legionella. Three additional results were weakly shown to be
Legionella but were still counted as positive samples out of an abundance of caution (Table 4).
Figure 4. DNA amplification with primers specific for Legionella. DNA in F1, F2 and F3 was from a
positive control.
Table 4. DNA Sequencing Confirmation of Legionella Species
Date Coupon
PCR plate
location Sequencing Results
May 19
th
, 2017 Influent A A2 Legionella sp.
June 22
nd
, 2017 Effluent B B11 Legionella sp.
July 31
st
, 2017 Influent B C5 Legionella sp.
August 30
th
, 2017
Influent A D3 Legionella sp.
Influent B D6 Legionella sp.
October 10
th
, 2017 Influent A F2 Legionella pneumophila
B-14
Modeling Results of Increased Temperature in Distribution
Several modeling scenarios were evaluated to determine the magnitude of impact from the recirculation
of the heat exchange effluent back to the drinking water distribution system. It is important to note that in
the model simulations, temperature was treated like a conservative dissolved constituent that would
simply respond to dilution effects; i.e., the temperature would increase proportionally if blended with a
warmer water, and then would remain unchanged until it reached a colder water. However, in reality
temperature is not conservative and the temperature of the water in the pipes will shift toward equilibrium
with the surrounding pipe and ground material. Thus, the temperature results that were modeled are
highly conservative and do not take into account any cooling that would naturally occur in distribution.
Thus, results indicated that when a significant portion of heat exchange effluent were re-circulated
through the local distribution system and back to the influent, temperature increase could build up more
than a single-pass temperature increase, and might result in water quality deterioration associated with
higher temperatures.
Additional model scenarios were therefore tested and showed that the re-circulation temperature effects
could be readily minimized or eliminated by increasing flow through the impact area. Figure 5 shows
modeled results of the temperature increase when a nearby well located approximately 0.4 miles north is
turned on (Well 10 Lynbrook-Hawthorne Ave, pumping about 1.7MGD). With most of the water for the
school originating from Well 10, the model estimated minimal short-circuiting, and the first downstream
customer having a temperature increase of less than 3°C, i.e., the temperature increase after a single pass
of the heat exchanger. The daily difference in influent and effluent temperature (Figure 2B) was at 3
o
C or
greater in 41.5% instances over 183 days of monitoring.
The actual system design minimizes the risk of warmer effluent water recirculating back to the heat
exchange system. Once the water is returned to the distribution system, it will be pushed to the streets to
the north of the school and would have to travel through one mile of pipe, which is four feet below the
ground, before it could reach the intake of the heat exchanger again. We assume the temperature of the
water will return to ambient ground water temperature as it travels back through the pipe.
The system has controls in place to maintain no more than a 2.78 degree Celsius (five degree Fahrenheit)
change in temperature between the influent and effluent. Once the effluent is discharged to the
distribution main, the SCADA system will automatically monitor the temperature change between the
influent and effluent and control the pumps to increase or decrease the water flow through the heat
exchanger to maintain the desired delta T. For example, on a particularly hot day, the pumps will speed
up to increase the amount of water flowing through the heat exchanger to minimize the increased
temperature of effluent. Under the pilot phase where water is discharged to an aquifer instead of the
distribution system, the rate of flow through the heat exchanger is fixed and adjusted manually. The
pumps generally run at 220 gallons per minute (gpm) have the ability to increase the flow rate up to 350
gpm.

B-15
Figure 5. Modeled temperature impact of introducing heat exchange effluent to distribution with Well 10
online-color coded maximum temperature increase in the impact area
Summary and Conclusions
Under the single pass testing, the heat exchanger increased temperatures by an average of 2.9
o
C, but the
disinfectant residual was maintained. Biofilm formation and related microbial activity was monitored
with ATP as well as heterotrophic bacteria growth and did not significantly change in the post heat
exchanger water. Legionella was not detected using the culture method widely used by the industry as the
gold standard. The more sensitive molecular method rarely detected Legionella in the water. Even then,
the detected pieces of Legionella DNA were in very low concentrations as reflected by the low gene copy
number. Only one influent sample at the testing site had L. pneumophila. When present, Legionella was
not amplified in the heat exchanger.
Hydraulic modeling evaluated the heat exchanger impact on temperature increase after re-introducing its
effluent into the distribution system. Under average/maximum day demand conditions, temperature
increase at the first downstream customer had potential to build up more than the single-pass temperature
increase due to re-circulation, which might cause deterioration of water quality. However, with
operational adjustment (turning on Well 10) or capital improvement (re-introducing the effluent to a
nearby 24-inch transmission), re-circulation could be readily minimized or eliminated. Nowhere within
the distribution would system temperature increase more than 3
o
C. In conclusion, based on the evidence
collected to date the heat exchanger does not appear to increase the risk of Legionella amplifying in the
outflow line or impact distribution system temperatures once the water is re-introduced into the
distribution system.
Plant5
Plant24
Well10
SchoolandImpactArea
withIncreasedTemperature
Temperature
Increase(°C)

B-16
References
Grattard F, Ginevra C, Riffard S, Ros S, Jarraud S. 2006. Analysis of the genetic diversity of Legionella
by sequencing the 23S-5S rRNA region- from phylogeny to direct identification of isolates at the species
level from clinical specimens. Microbes and Infection 8:73-83.
LeChevallier M.W., O.D. Schneider, L.A. Weinrich, P.K. Jjemba, P.J. Evans, J.L. Hooper, and R.W.
Chappell (2015) An Operational Definition of Biostability in Drinking Water. Water Research
Foundation Report# 4312b, Denver, Colorado. (http://www.waterrf.org/PublicReportLibrary/4312b.pdf).
Promega (2009) Technical Bulletin – BacTiter-Glo
TM
Microbial Cell Viability Assay. Part TB337.
Velten S., F. Hammes, M. Boller, and T. Egli (2007) ATP measurement as a means for directly
estimating active biomass. Promega Notes 97:15-17.
Grattard
B-17
Appendix
Methodology
Pipe loops installed in July 2015 on the inlet and outlet side of the heat exchanger have ports
where two coupons (i.e., A and B) are inserted and retrieved as needed (Figure A-1). Results from
the first phase were summarized by ORNL in a previous report and are not covered in this report.
There was a period of eight months from when the first phase ended and when the second phase
began. The coupons were left in place (with the water flowing) from the end of the first phase to
the beginning of the second testing phase. This provided an opportunity to harvest biofilms
developed over eight months as compared to the typical 30-day duration. Additional monthly
coupon harvestings were conducted in June, July, August and October 2017 and shipped to the
laboratory overnight on ice. Residual chlorine disinfect, pH and water temperature were collected
using an online monitor once every hour.
In the laboratory, the biofilm was scrapped off each coupon and suspended in 30 mL phosphate
buffer (Figure A-2). Aliquots were used to determine microbial activity (using adenosine
triphosphate-ATP as a surrogate), heterotrophic bacteria (HPC), and Legionella (Figure A-3).
Adenosine triphosphate (ATP) measurements depended on BacTiter-Glo
TM
Reagent with a
proprietary thermostable luciferase enzyme isolated from fireflies. The enzyme requires energy
from ATP to produce light detected by luminescence (Veltens et al., 2007; Promega, 2009). It
was determined in triplicate aliquots of 100 μL biofilm suspension by adding to an equal volume
of BacTiter-Glo™ Reagent (Promega, Madison, WI). The mixture was incubated at 30 °C for 1.5
min with periodic mixing every 30 seconds. Luminescence (relative light units) was measured
exactly 30 s using the GloMax
TM
20/20 Luminometer (Promega, Madison, WI). The
luminescence value was converted to ATP concentrations based on a calibration curve obtained
by spiking serial dilutions of a 10mM ATP stock incubated with the biofilm suspension which
had been inactivated by heating at 60
o
C (21 hours) and then treated as any other sample (Veltens
et al., 2007; LeChevallier et al., 2015). The final ATP concentration was related to the surface
area of the coupon and formation rate per day determined based on the number of days each
coupon had been in place. Heterotrophic bacteria in the biofilm were determined in duplicates
using the culture spread-plate method (Standard Method 9215C [Eaton et al., 2005]) on R2A
agar. To break up clumps, the sample was vortexed at maximum speed for 30s and tenfold
dilutions plated. The plates were incubated at 22±1
o
C for one week. HPCs were expressed on a
per unit area (i.e., HPC/mm
2
) of the coupon.

B-18
Figure A-1. Coupons (A) and coupon assembly in the pipeloop (B) in relation to water flow
Figure A-2. Harvested biofilm suspension
A
B

B-19
Duplicate 100 µL aliquots of the biofilm suspension were plated on BCYE agar supplemented
with GVPA (Oxoid) and a Legionella agar enrichment (BD Difco, Sparks, MD; Figure A-3). To
ensure detection of low levels of Legionella, another aliquot of 20 mL biofilm suspension was
filtered through a 0.2 µm 47-mm diameter polycarbonate filter. The filter was aseptically
removed and inserted into 10 mL sterile water and vortexed at high speed to re-suspend the
bacteria. To eliminate non-Legionella organisms during this selective growth process, 1 mL of
the sample was pretreated with acidified potassium chloride (0.2 M KCl-HCl reagent; pH = 2.2)
for 15 minutes at room temperature. A 0.1 mL aliquot of the mixture was spread-plated on BCYE
agar supplemented with GVPA (Oxoid) and a Legionella agar enrichment (BD Difco, Sparks,
MD). The plates were incubated at 36.5°C with 2.5% CO
2
and 94% relative humidity. Growth on
the plates was monitored for up to 10 days.
DNA was extracted from an aliquot of the biofilm suspension using the QIAamp® Circulating kit
(Qiagen) as specified by the manufacturer. DNA was eluted with water (final volume of 50 µL)
and frozen (-20
o
C) until qPCR. Amplification was conducted targeting the variable 23S-5S gene
using primers ISR-F 5’- TGAAGCCCGTTGAAGACTAC-3’ and ISR-R 5’-
GGAAGCCTCACACTATCAT-3’ described by Grattard et al. (2006) synthesized by TIB
MolBiol LLC (Adelphia, NJ). The number of copies were quantified with a G-Box synthesized
by Integrated DNA Technologies, Iowa. PCR was conducted by a LightCycler 480 (Roche
Diagnostics, Indianapolis, IN) in 20 μL aliquots through 35 cycles of denaturation (15s at 94
o
C),
annealing (25s at 55
o
C) and extension (25s at 72
o
C).
All PCR-positive products were examined on a gel to confirm the expected size of 300 base pairs.
Because most bands on the gel were very faint (indicating low concentrations of the target DNA),
the PCR products were re-amplified and then sequenced by TIB MioBiol LLC using the same
type of set of primers. BLAST searches were performed on all valid sequences using the National
Center for Biotechnology Information database (www.ncbi.nlm.nih.gov) to determine their
identity.

B-20
Figure A-3. Sample processing and workflow
during the second testing phase
30mL biofilm solution (vortexed for 1 minute)
Direct plate 0.1mL on
BCYE w/GVPA
Legionella detection and enumeration
ATP analysis (for
microbial
activity) with
Luminometer
Heterotrophic
bacteria (HPC) on
R2A agar
(Duplicates)
Filter 20mL onto 0.2 μm
p
ol
y
carbonate filte
r
Transfer to 10mL sterile
water and vortex (1minute)
Direct plate 0.1mL on
BCYE w/GVPA
(D li )
Incubate at 37
o
C (2.5% CO
2
; 10 days)
Extract DNA
(4mL sample)
Conduct qPCR
Sequence qPCR
positive and
uncertain
products
Incubate at 22
o
C (7 days)
B-21
Model Simulation of Water Temperature Increase in Distribution
The above laboratory tests were conducted to evaluate water quality impact after a single pass through the
heat exchanger. The follow up question was how re-injecting the heat exchanger effluent to the
distribution system would impact distribution water temperature. The increased temperature in the heat
exchanger effluent would dissipate via dilution and diffusion with ambient distribution environment, after
re-injecting to the distribution system. It is important to prevent or minimize the heated effluent
recirculating to the suction side of the heat exchanger, which may cause heat build-up and eventually
deteriorate water temperature quality.
To address these questions, existing system’s hydraulic model was used to simulate the dissipation of the
increased temperature from the heat exchange effluent. The model was last updated and calibrated using
WaterGEMS Connect (Bentley – Watertown, CT) by a consultant Mott MacDonald in 2016 (model build
report available upon request). A project site map and a screenshot of the modeling program are presented
in Figure A-4. The heat exchanger was modeled as a flow controlled valve and a pump with a flow
setting of 300 gpm. The increased temperature was set at 2.91°C based on the average temperature
increase observed at the facility in May to October 2017. The influent of the heat exchanger was
simulated at the 12-inch main on Horton Avenue, while the discharge effluent was simulated on the 6-
inch main on Trafalgar Square. A total of about 520-feet 6-inch main was assumed to be installed to take
water from Horton Avenue, through the heat exchanger, and then discharge at Trafalgar Square.

B-22
Figure A-4. Project site map (A) and model representation of the heat exchanger and the distribution
system (B)
Well#10
B
A
24‐Inch
Transmission
Discharge6‐Inch
onTrafalgarSq
Suction12‐Inch
onHortonAve
HeatExchanger
HeatExchanger
24‐Inch
Transmission
Well#10
B-23
Dissipation of increased temperature is mainly through dilution and diffusion with the ambient
distribution environment. To simulate diffusion, a first-order decay model was assumed with a decay
constant set at -3.0°C/day, i.e., increased temperature drops to about half after four (4) hours traveling
within the distribution system. This was considered low to be conservative, assuming minimal heat
diffusion within the distribution system. The actual heat diffusion in distribution systems can be highly
variable depending on pipe materials, pipe depth, soil conditions, and groundwater levels. The dilution of
the increased temperature via mixing with distribution water depends on system hydraulics near the
suction and discharge side of the heat exchanger. The following scenarios were simulated to evaluate the
impact of different system conditions on dilution/mixing:
1. Baseline - existing average day demand (ADD) conditions (~27MGD) with Well 10 offline;
2. Existing maximum day demand (MDD) conditions (~48MGD) with Well 10 offline;
3. Existing ADD conditions (~27MGD) with Well 10 online;
4. Existing ADD conditions (~27MGD), Well 10 offline, and heat exchanger effluent discharge to
the 24-inch transmission on Peninsula Blvd and Rockaway Ave.

B-24
Kendra F. Morris Email communication to Ellen D. Smith, February 19, 2018.
From: Kendra Morris <[email protected]>
Sent: Monday, February 19, 2018 7:58 PM
To: Smith, Ellen D.
Cc: Liu, Xiaobing; Ben D Stanford; Patrick K Jjemba; Don Wieczenski
Subject: FW: American Water NY Geothermal Pilot - Draft Report Comments
Ellen,
Please see the responses to your two questions below. We are happy to get on the phone to discuss
further.
1. Question: What was the methodology used on the quantitative PCR test? Was this a
commercially available product? How was the base qPCR done?
Response: The PCR protocol was not from a commercial kit but rather conducted using
published sequences targeting the variable 23S-5S gene using primers ISR-F 5’-
TGAAGCCCGTTGAAGACTAC-3’ and ISR-R 5’- GGAAGCCTCACACTATCAT-3’ described
by Grattard et al. (2006). The quantification (i.e., qPCR) was based on the complete sequence of
Legionella pneumophila strain Philadelphia-1 (NCBI GenBank: CP013742.1) in a synthesized
genetic block (G-block) whose sequence is shown below. Concentration of this 336 bases g-block
stock contained 2.76 × 10
10
copies (see conversion at http://cels.uri.edu/gsc/cndna.html). Tenfold
dilutions of the G-block were loaded into triplicate wells F10,F11,F12 (0.1ng/uL), G1,G2,G3
(0.01ng/uL), G4,G5,G6 (0.001ng/uL), G7,G8,G9 (0.0001ng/uL), and G10,G11,G12
(0.00001ng/uL) and provided the standard curve used to calculate the number of copies in actual
samples loaded in rows A through E. Wells F1,F2,F3 had DNA from a known Legionella strain
(i.e., positive control).
2. Question: On Figure 4 DNA amplification with primers (page 7): What were rows F, G and H?
For row F, why are there negative results for the positive control sample?
Response:
F1,F2,F3 had DNA from a Legionella strain (Positive control)
F4,F5,F6 had primers (i.e., everything needed for PCR) but NO DNA added (Negative control)
F7,F8,F9 did not have any sample or primers (i.e., were empty)
F10,F11,F12 had the highest concentration of G-block (see response to #1 above)
Row G (other G-block dilutions; see contents outlined in response to #1 above)
Row H did not contain any samples or primers (i.e., all wells in row H were empty)
Regards,
Kendra
-------------------------------------------------------------------------------------
Kendra F Morris
O 856.359.2091 |C 609.315.5079 | Kendra.Morris@amwater.com
