VIRAL HEPATITIS SURVEILLANCE
AND CASE MANAGEMENT
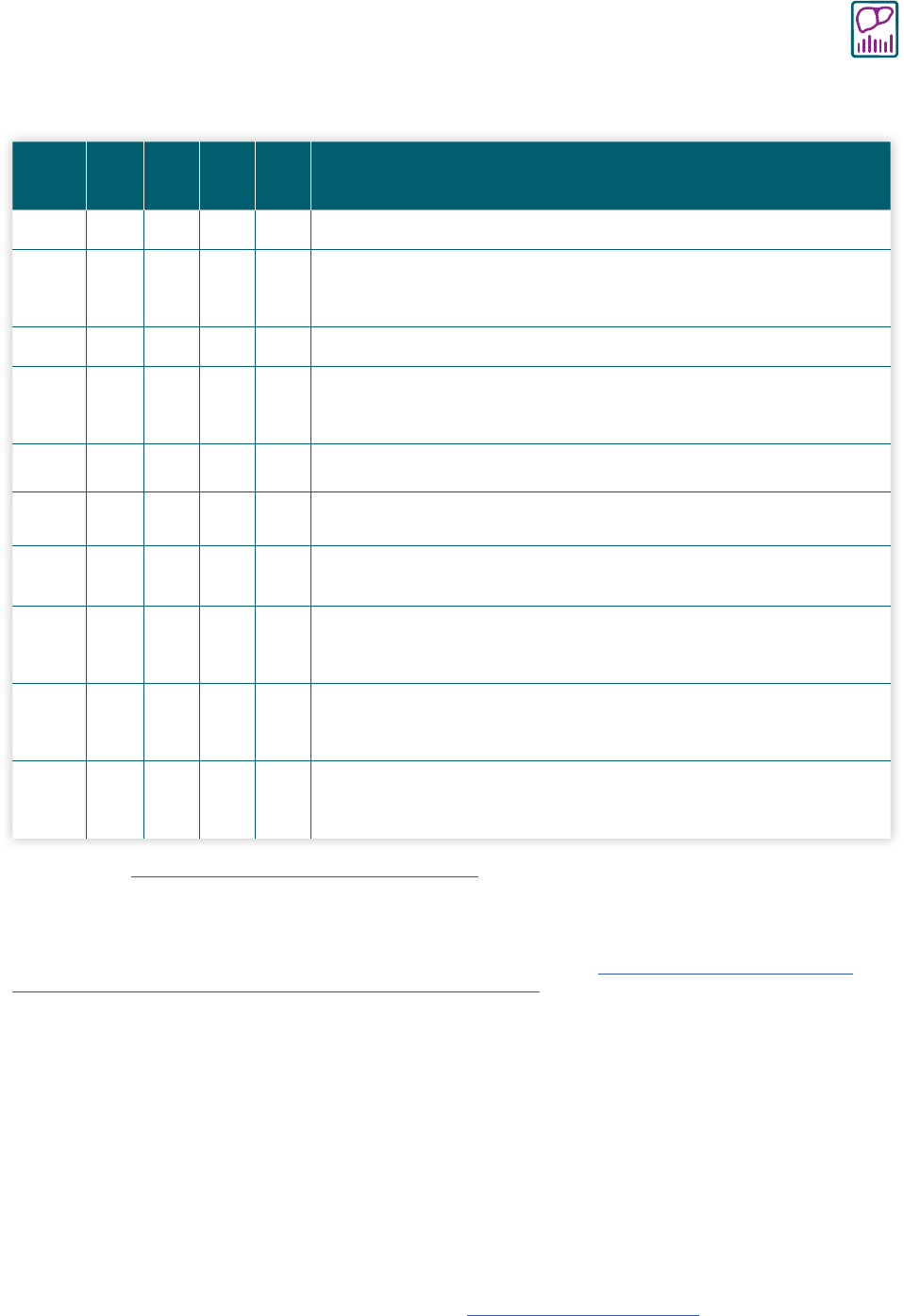
Table 3-1. Interpretation of hepatitis B laboratory results
HBsAg
Total
anti-
HBc
Anti-
HBc
lgM
Anti-
HBs
HBV
DNA
Possible Interpretation*
— — — — — Never infected; susceptible if never vaccinated or vaccine failure
+
— — —
+
or
—
Early acute infection (if HBV DNA is positive); transiently positive for
HBsAg after vaccination (if HBV DNA is negative)†
+ + +
—
+
Acute infection
—
+ +
+
or
—
+
or
—
Acute resolving infection; “window period” if anti-HBs is negative
—
+
—
+
— Recovered from past infection and immune
+ +
— —
+
Chronic HBV infection
— — —
+
—
Immune from vaccination; passive anti-HBs transfer after hepatitis B
immune globulin administration
—
+
— —
+
or
—
Isolated total anti-HBc positive‡
—
+
or
—
—
+
or
—
+
Occult HBV infection
§
+
or
—
§
+
+
or
—
+
or
—
+
Possible HBsAg mutant infection
Table modified from https://www.cdc.gov/mmwr/volumes/67/rr/pdfs/rr6701-H.PDF.
Abbreviations: – = negative; + = positive; anti-HBc = antibody to hepatitis B core antigen; anti-HBs = antibody to hepatitis B surface antigen; HBsAg =
hepatitis B surface antigen; HBV DNA = hepatitis B virus deoxyribonucleic acid; IgM = immunoglobulin class M.
*Ingestion of high levels of biotin can significantly interfere with certain commonly used biotinylated immunoassays and cause false-positive or
false-negative laboratory test results. The US Food and Drug Administration (FDA) is investigating thresholds associated with false-positive and
false-negative tests. This section will be updated as more information becomes available. Reference: https://www.fda.gov/medical-devices/safety-
communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication.
†People who receive hepatitis B vaccine might be transiently positive for HBsAg, with reports of transient positivity 18 days post-vaccination
(56)
.
Retesting of patients who are positive for HBsAg shortly after hepatitis B vaccination at a later time is needed to determine the true HBV infection
status.
‡Could result from:
• Loss of anti-HBs after past resolved infection. HBV DNA is negative.
• False-positive total anti-HBc, i.e., susceptible. HBV DNA is negative. To resolve the ambiguity of a false-positive total anti-HBc result, test a
follow-up sample 4–8 weeks later. If found positive, interpret as a resolved infection. If negative, interpret as false-positive.
• Passive maternal transfer of total anti-HBc to infant born to a HBsAg-positive gestational parent for up to 24 months. HBV DNA is negative.
• Occult HBV infection. HBV DNA is positive, typically at low levels. Anti-HBs might or might not be positive.
• HBsAg mutant infection. HBV DNA is positive, typically at high levels. Anti-HBs might or might not be positive.
§
HBsAg mutants will not be detectable if testing was performed using an older assay that cannot detect HBsAg mutants. HBsAg mutant strains can
be detected by some HBsAg assays that first became available in the United States in 2015, including Abbot ARCHITECT instrument, ETI-MAK-2 PLUS,
and Siemens Advia Centaur XP or XPT instrument. Though specimens should be tested using an assay that can detect HBsAg mutants, older HBsAg
assays that cannot detect HBsAg mutants remain available. Reference: Apata I W, Nguyen D B, Khudyakov Y, et al. Hepatitis B virus mutant infections in
hemodialysis patients: A case series. Kidney Medicine 2019; 1(6): 347-353. DOI: https://doi.org/10.1016/j.xkme.2019.07.011.
