
DEPARTMENT OF MEDICAL
LABORATORY SCIENCE
BACHELOR OF MEDICAL LABORATORY SCIENCE
(BMLS) PROGRAMME
Yaba, Lagos
Table of Contents i
Title Page ii
WELCOME NOTE FROM THE HEAD OF DEPARTMENT iii
ADMINISTRATIVE STRUCTURE OF THE DEPARTMENT iv
LIST OF ACADEMIC STAFF v
INTRODUCTION 1
PHILOSOPHY 2
AIMS AND OBJECTIVES 3
ADMISSION REQUIREMENTS 4
COURSE DURATION 5
EVALUATION OF STUDENTS 5
LABORATORY POSTINGS 5
ATTENDANCE POLICIES 5
PASS MARKS 6
CONTINUOUS ASSESSMENT SCORE 7
EXAMINATION SCORE 7
MOVEMENT OF STUDENTS BETWEEN ACADEMIC LEVELS 7
WITHDRAWAL 8
COURSE CREDIT SYSTEM 8
GRADE POINT AVERAGE (GPA) 8
CUMULATIVE GRADE POINT AVERAGE (CGPA) 9
GRADE POINT AVERAGEAND CUMULATIVE GRADE POINT AVERAGE 10
INDEXING/ REGISTRATION OF STUDENTS WITH THE MEDICAL LABORATORY
SCIENCE COUNCIL OF NIGERIA 10
FIRST AND FINAL PROFESSIONAL EXAMINATIONS 11
The Final Professional Examination consists of:- 12
INDUCTION OF GRADUATES 13
DRESS CODE 14
INTERNSHIP PROGRAMME 14
EXAMINATION MISCONDUCT IN ACCORDANCE WITH THE
UNIVERSITY REGULATIONS
14
PENALTIES FOR EXAMINATION MISCONDUCTS 15
GRADUATION REQUIREMENTS 17
Minimum Credit Load per Semester 18
FIRST YEAR (100L) COURSES FOR THE DEPARTMENTOF MEDICAL
LABORATORYSCIENCE 19
COURSE OUTLINE: 100 LEVEL MEDICAL LAB SCIENCE
FIRST SEMESTER
HAEMATOLOGY & BLOOD TRANSFUSION SCIENCE (SPECIALTY)FIRST
SEMESTER 29
HISTHOPATHOLOGY (SPECIALITY) 31
FIRST SEMESTER
MEDICAL MICROBIOLOGY (SPECIALITY) 32
FIRST SEMESTER
DESCRIPTION OF COURSES 35
AWARDS AND ACHIEVEMENTS 66
LINKAGES AND COLLABORATIONS 68
PENALTIES FOR EXAMINATION MALPRACTICES 69
WELCOME NOTE FROM THE HEAD OF DEPARTMENT
I sincerely wish to welcome all of you to the Department of
Medical Laboratory Science. We are highly excited to be partner
with you as you participate in an exciting educational journey of
discovery. I would like to extend a warm welcome as you begin
what promises to be an exciting journey to a noble profession.
You have made the right choice of University in Trinity
University and Department of Medical Laboratory Science. You
will be working with a world-class departmental staff dedicated
to helping you get the best of university education. You have
entered the gateway to a global education that will prepare you to
succeed in an increasingly competitive international marketplace.
Indeed, you are among a generation studying to acquire world
class knowledge. In this department and in the University, you
will meet the people who will become your life vision assistants
and mentors. Take advantage of the many services available to
help you achieve your full potential both on site or online.
All of us at the Department of Medical Laboratory Science and
Trinity University at large are strongly committed to supporting
your success. In return, we know you will study hard, seek out
and respect the opinions of others, enjoy the rich variety of
activities that the university has to offer and find the
opportunities to give back to your communities. Much of your
success in your journey through life will depend on the efforts
and the choices you make now. We aim to nurture excellence and
provide you with an exceptional learning experience that will
enable you to become an independent thinker.
I am confident that your time in Trinity University and Medical
Laboratory Science Department will be full of rewarding
experiences. Thank you for being a special member of the
Department of Medical Laboratory Science.
Dr. (Mrs.) R. M. Kolawole

ADMINISTRATIVE STRUCTURE OF THE
DEPARTMENT

vi
LIST OF ACADEMIC STAFF
S/N
NAME OF STAFF
RANK/DESIGNATION
SALARY SCALE, DATE OF
FIRST APPOINTMEN
F/T
QUALIFICATION, DATES
OBTAINED,
SPECIALISATION,
MEMBERSHIP OF
PROFESSIONAL
ASSOCIATION AND
NUMBER OF
PUBLICATIONS
POST QUALIFICATION,
WORK/TEACHING
EXPERIENCE AND DATE,
POST HELD AND THE
ORGANISATION
COURSE/SUBJECT TAUGHT
GENDER
OTHER
RESPONSIBILITIES/INTERE
ST IN CURRICULULAR AND
EXTRA – CURRICULAR
ACTIVITIES.
1
Dr. Kolawole
R. M.
Ag. HOD/
Senior
Lecturer/05
Step 1
FT
(Sab
batic
al)
Ph.D, M.Sc.,
AMLS,
MLSCN/AMLSN,
Medical
Microbiology
3 years
Medical
Microbiology ,
Mycology and
Parasitology
F
Ag.HOD/
300 Level
Course
Adviser
2
Dr.
Bartholomew
Ifionu
Senior
Lecturer/05
step 1
FT
Ph.D, M.Sc.,
FIMLT, AIMLT,
MLSCN/AMLSN,
Chemical Pathology
6 years
Chemical
pathology,
Introduction to
Medical
Laboratory
Science and
Posting
M
200 Level
Adviser
3
Osiagwu
Daniel
Lecturer 11/02
step 11
FT
M.Sc. AIMLT
3 years
Histopatholog
y/Cytopatholo
gy
M
400 Level
Adviser
4
Mrs. Obiageri
Okeoma
LII/02 Step 1
FT
M.Sc., BMS,
AMLSCN, PGD
1 yr
Haematology/
Chemical
pathology
F
100 Level
Adviser
5
Prof. M. A.
Muhibi
Professor/
50% of 07
step 1
Visit
ing
Ph.D, FWAPCMLS,
FMLSCN, M.Sc.,
PGD, AIMLT
9 years
Haematology
and Blood
Transfusion
Science
M
-
6
Prof. M. F.
Olaniyan
Professor/
50% of 07
step 4
Visit
ing
Ph.D, FWAPCMLS,
FMLSCN, M.Sc.,
PGDE, AIMLT,
Cert. Imm
14 years
Chemical
Pathology and
Immunology
M
Consultant
7
Dr. T. Y.
Raheem
Reader/50%
of 06 step 1
Visit
ing
Ph.D, FWAPCMLS,
FMLSCN M.Sc.,
PGD, AIMLT, Cert.
Imm
9 years
Medical
Microbiology
and
Immunology
M

vii
8
Dr. Adesina,
Opeyemi
Senior
Lecturer1/ 05
step 1
Visit
ing
Ph.D (2016), M.Sc.
(2009), B.Sc.,
AIMLT, FMLSCN.
3 years
Haematology
and Blood
Transfusion
Science
F
9
Dr. Christian
Enwuru
Lecturer1/ 04
step 1
FT
Ph.D 2019
M.Sc 2006
AMLS 1999
3 years
Medical
Microbiology
& Molecular
Biology
M
LIST OF LABORATORY STAFF

viii
SN
NAME
QULIFI
CATION
AREA
OF
SPECIA
LIZATI
ON
RANK
GENDE
R
EMPLO
YMENT
STATUS
1
Mr. Emmanuel
Fasela
M.Sc. Molecular
Diagnostic,
FIMLS
Haematology,
AIMLS
Chemical
Pathology
Haematology and
Chemical
Pathology
Chief
Medical
Laboratory
Scientist
M
Full Time.
2
Mr. Samuel
Akindele
M.Sc. Medical
Microbiology,
FMLS
Parasitology
Medical
Microbiology
Assit. Chief
Medical
Laboratory
Scientist
M
Full Time
3
Mrs. Blessing
Adakole
BMLS
Histopathology
Histhopatology
Principal
Medical
Laboratory
Scientist
F
Full Time
4
Mr. Christian
Isaac Ubi
BMLS
Haematology
Haematology
Medical
Laboratory
Scientist 1
M
Full Time
6
Olaniyan
Tolulope
Busayo
MLT
NA
Senior
Medical
Laboratory
Technician
F
Full Time
6
Olayode
Christopher
Olasunkanmi
MLT
NA
Medical
Laboratory
Technician I
M
Full Time
7
Kareem Titilayo
MLT
NA
Medical
Laboratory
Technoiian I
F
Full Time
8
Taiwo
Oluwadamilola
MLT
NA
Medical
Laboratory
Technician I
M
Full Time

ix
LIST OF ADMINISTRATIVE STAFF
S/N
NAME
RANK
QUALIFICATION
GENDER
EMPLOYMENT
STATUS
1
Mr. Sunday
Abiola
Assistant
Registrar
(Faculty
Office)
B.Sc.
Administration
M
Full Time
2
Miss
Alende
Lynda
Administrative
Officer
(Faculty
office)
HND, Hospitality
and Tourism
F
Full Time
3
Mr.
Fatimeyin
Ayotunde
Administrative
Officer
(Departmental
Office)
HND, Business
Administration
M
Full Time
4
Mrs. Helen
Odion
Administrative
Assistant
(Departmental
Office)
ND, Business
Administration
F
Full Time
1
INTRODUCTION
The Medical Laboratory Science Council of Nigeria, which
was established by Act. 2003 Cap 114 Laws of the Federation
of Nigeria, regulates the practice of Medical Laboratory
Science in Nigeria. The science entails the theory and practice
involving the analysis of human or animal tissues, body fluids,
excretions, production of biologicals, design and fabrication of
equipment for the purpose of medical laboratory diagnosis and
research. The subject areas include medical microbiology,
chemical pathology, haematology, blood transfusion science,
virology, histopathology, histochemistry, immunology,
cytogenetics, exfoliative cytology, parasitology, forensic
science, molecular biology, laboratory management, or any
other related subject as may be approved by the Medical
Laboratory Science Council of Nigeria and the University
Senate. The profession combines the use of scientific
instruments and techniques with the application of scientific
knowledge in the performance of complex analytical
procedures on tissue specimens, blood samples and other body
fluids. These diagnostic tests and procedures are carried out by
trained health professionals called Medical Laboratory
Scientists. The roles of Medical Laboratory Scientists entail,
and are not limited to the, diagnosis, prevention of diseases,
disease surveillance and health promotion. As a highly skilled
and disciplined member of the healthcare team, the Medical
Laboratory Scientist is expected to ensure a robust inter-
professional relationship and collaborate with other members
of the health team. Good professional practice and teamwork
should remain the guiding principles of the profession.
The programme of the Department is Bachelor of Medical
Laboratory Science (BMLS) being regulated by National
Universities Commission (NUC) and by the Medical
Laboratory Science Council of Nigeria (MLSCN). The
Department operates in the Faculty of Basic Medical Sciences.
Students at their first year (100 level ) are trained under the
2
Faculty of Science in Basic Sciences and Faculty of Arts,
Management and Social science in General studies. At the 200
level, students are introduced to Medical Laboratory Science.
The third year of the programme marks the beginning of the
professional training as students are engaged in the classroom
for lectures as well as in the Hospital laboratory for the
professional or practical training. At the fourth-year students
are taught the basics in all the special areas of Medical
Laboratory Science. At the end of 400 level programme,
successful students are presented for the First professional
examination, where the Medical Laboratory Science Council of
Nigeria is expected to be represented by an Assessor or
Observer. At the fifth year, students break into 4 core or
specialised areas of Medical Laboratory Science, namely:
Medical Microbiology, Clinical Pathology,
Haematology/Blood Transfusion Science and
Histopathology/Histochemistry. At the end of the fifth year,
suitable students are presented for Final professional
examination, where the Medical Laboratory Science Council of
Nigeria is expected to be represented by an Assessor or
Observer. Upon the successful completion of the 5-year
training, graduates of the programme are inducted and issued a
provisional license by the regulatory body. Following a
successful induction into the profession, graduate of Medical
Laboratory Scientists must also undergo a compulsory
internship in any accredited laboratory before they are
mobilised for National Youth Corps Scheme.
PHILOSOPHY
The broad philosophy of the Bachelor of Medical Laboratory
Science degree programme includes:
a) Provision of sound academic and professional
background for the production of Medical Laboratory
3
Scientists who would be capable of working anywhere in
Nigeria.
b) Production of Medical Laboratory Scientists who
would satisfy internationally recognizable standards and who
could undertake further training towards specialization.
c) Production of Medical Laboratory Scientists with
sufficient management ability to play a leadership role and
entrepreneurship in employing others, establishing self, and
also in training and general practice of medical laboratory
sciences.
AIMS AND OBJECTIVES
i) To instill in the student a sense of enthusiasm for the
profession; an appreciation of its application in
different contexts (in areas such as general medicine,
food and beverages, pharmaceutical industries, utility
departments such as water corporations and research
institutions.
ii) To involve the students in an intellectually stimulating
and satisfying experience of learning, studying and
research.
iii) To provide students with a broad and balanced
foundation of medical laboratory knowledge and
practical skills; performing effectively in clinical
diagnostic services, academia and quality assurance;
and function independently or in collaboration with
other members of the health team in the care of
individuals and groups at all levels of health care.
4
iv) To develop in students, the ability to apply their
medical laboratory knowledge and skills to the
solution of theoretical and practical problems in
laboratory medicine.
v) To develop in students through an education in
medical laboratory sciences, a range of transferable
skills of value in medical and non-medical
employment.
vi) To provide students with a knowledge and skills base
from which they can proceed to further studies in
specialised areas involving medical sciences.
vii) To generate in students, an appreciation or the
importance of medical laboratory science in an
industrial, economic, health and social context.
viii) To generate students with the ability to produce
biological and diagnostic reagents as well as being
able to fabricate and maintain laboratory equipment.
ix) To also empower graduates of Medical Laboratory
Science with skills that will enable them to engage in
income yielding ventures i. e. a re-orientation from
the ‘take- a job’ mentality to the ‘make-a-job’
mentality.
ADMISSION REQUIREMENTS
a. Admission through UTME:
i. Candidates must satisfy the minimum University
requirements for admission and are therefore to obtain
credits in five subjects of English Language,
5
Mathematics, Biology, Physics and Chemistry in SSCE
or its equivalent in not more than two sittings.
ii. In addition to the above, the candidate must have an
acceptable score in the Unified Tertiary Matriculation
Examination (UTME) conducted by JAMB.
b. Admission by Direct Entry:
i. Candidates holding three GCE A/L in Biology,
Chemistry and Physics plus O/L Credits in five
subjects of English Language, Mathematics, Biology,
Physics and Chemistry.
ii. Medical Laboratory Technician certificate of the
Medical Laboratory Science Council of Nigeria
iii. Candidate who transfers from other faculties of the
University with relevant prerequisites.
iv. B.Sc. degree in relevant science disciplines like
Zoology, Microbiology, Anatomy, Physiology and
Biochemistry,
v. HND in Microbiology, Biochemistry and
Pharmacology fields as approved by the senate.
c. Transfer of students from other Universities:
Suitability and placement will be determined by the
quality of the transcripts as approved by the senate of the
University, in line with the guidelines of the professional
and academic regulatory bodies
COURSE DURATION
The Bachelor of Medical Laboratory Science degree
programme shall run for five (5) years for UTME candidates
and four (4) years for direct entry candidates.
EVALUATION OF STUDENTS
Each MLS Course taught in the BMLS Programme at the
University may be evaluated for grading with the use of one or
several of the following criteria:
i. Written examinations which include problem solving: -
6
a. Essay: Six (6) questions to attempt four (4).
b. Multiple choice question (MCQ) 40 questions (5 parts)
to attempt all.
ii. Laboratory presentations or demonstrations to the class of
exercises/techniques.
iii. Laboratory Reports.
iv. Case studies/Laboratory logbook.
v. Continuous assessment tests.
No students shall be allowed into the examination venue if
he/she has not fulfilled the mandatory posting.
ATTENDANCE POLICIES
1. Attendance is compulsory and absences from class and/or
laboratories will affect student’s final grade. Missed
laboratory work and/or examinations must be completed.
2. Since sample procurement is difficult, laboratory absences
are particularly difficult, to make up 75% attendance is a
prerequisite to sit exams. Absence from laboratory postings
is tantamount to carry over of posting. Students are
therefore advised not to miss any laboratory session.
3. Protracted illness (three consecutive days or more) should
be reported to the head of Department promptly.
4. Students shall continue their laboratory posting during
holidays and this shall serve as their industrial attachment.
5. Final year students are to take compulsory call-duty in their
respective Discipline. They are to be attached to Medical
Laboratory Scientist on- call-duty. This shall be graded
part of the 75% attendance laboratory posting.
LABORATORY POSTINGS
Laboratory posting is compulsory for all Medical Laboratory
Science Students. For medical laboratory science students,
laboratory posting is in 300 – 500 levels and at least 75%
attendance is compulsory and is a prerequisite for writing the

7
examinations. The posting should be in a MLSCN certified
laboratory. Laboratory posting in accredited hospital or
medical/ research laboratory is to be between 2-3 days in a
week. Supervision and training in the hospital laboratory shall
be conducted by medical laboratory scientists in collaboration
with the academic staff of the Department appointed as
Consultants and must hold current practicing license issued by
Medial Laboratory Science Council of Nigeria.
PASS MARKS
Medical Laboratory Science, being a professional programme,
operates a modified course unit system
The pass mark for all 100 – 200 level courses shall be 45%
while the pass mark for all core Medical Laboratory Sciences
Courses (between 300 – 500 level) shall be 50%
CONTINUOUS ASSESSMENT SCORE
This shall constitute 40% of the total score of 100 for a course.
It shall be earned from assignments/presentations (20mks) and
tests (20mks)
EXAMINATION SCORE
This shall be 60% of the total mark of 100 for a course to be
earned from end of semester examinations.
Total
MOVEMENT OF STUDENTS BETWEEN ACADEMIC
LEVELS
Promotion from 100 to 200 Level
8
A student must not carry over more than 3 Compulsory courses
and must have earned not less than 24 ofunits of the total credit
load of the courses registered for the session to move from 100
to 200 level.
Promotion from 200 Level to 300 Level
A student must not carry over more than 4 Compulsory courses
and must have earned not less than 24 units of the total credit
load of the courses registered for the session to move from
200 to 300 level.
Promotion from 300 Level to 400 Level
A student must not carry over more than 4 Compulsory courses
and must have earned not less 24 unitsof the total credit load of
the courses registered for the session to move from 300 to 400
level.
A student who fail first professional examination at the end of
400 level shall not proceed to 500 level.
Promotion from 400 Level to 500 Level
A student who has any carry over at the end of 400 level shall
repeat the year (Full- Academic session) until failed courses are
passed
Requirements of Professional Examinations
Students must not carry any course over to the professional
examinations.
Repeat
9
Any student who fails to meet any of the movement criteria
shall repeat that level for not more than one year.
WITHDRAWAL
Astudent who fails after repeating a particular level SHALL be
advised to withdraw from the programme.
COURSE CREDIT SYSTEM
Course credit/Unit system must be employed in teaching all the
programmes. Pre-requisite courses must be passed before
students can register for higher level of a particular course
while non-requisite courses can be carried over.
GRADE POINT AVERAGE (GPA)
Performance in any semester is reported in Grade Point
Average. This is the average of weighted grade points earned
in the courses taken during the semester. The Grade Point
Average is obtained by multiplying the Grade Point average in
each course by the number of Credit Units assigned to that
course, and then summing these up and dividing by the total
number of Credit Units taken for the semester.
CUMULATIVE GRADE POINT AVERAGE (CGPA)
This is the up-to-date mean of the Grade Points earned by the
student in a programme of study. It is an indication of the
student’s overall performance at any point in the training
programme. To compute the Cumulative Grade Point Average,
the total of Grade Points multiplied by the respective Credit
Units for all the semesters are added and then divided by the
total number of Credit Units for all courses registered by the
student.
10
GRADE POINT AVERAGEAND CUMULATIVE GRADE
POINT AVERAGE
Classified: -
First class division 4.50 – 5.0
Second class upper division 3.50 – 4.49
Second lower division 2.40 – 3.49
Third class division 1.50 – 2.39
Pass 1.0 – 1.49
Scores Grade Credit point
70 – 100% A 5
60 – 69 B 4
50 – 59 C 3
45 – 49 D 2
40 – 44 E 1
0 – 39 F 0
INDEXING/ REGISTRATION OF STUDENTS WITH
THE MEDICAL LABORATORY SCIENCE COUNCIL
OF NIGERIA
This is carried out on-line and the portal will open for the
registration of students between JULY and SEPTEMBER
every year. Indexing of students is carried out on or before the
completion of 300 level programme. It involves:
i) Completion of ONLINE student Registration
Enrolment Form by the Head of Department or as may be
delegated and should be a registered member of MLSCN with
current practicing licence.
11
ii) Payment of prescribed fees.
iii) Presentation and screening of credentials for eligibility.
iv) Eligible students are then enrolled as student Medical
Laboratory Scientists with student registration number.
v) Students’ enrolment letters are sent through the Head
of Department
vi) Students that fail the screening would be advised to
withdraw from the programme forthwith.
FIRST AND FINAL PROFESSIONAL EXAMINATIONS
Students are examined in two phases for:-
First Professional Examination (400 level) and Final
Professional Examination (500 level). To pass the examination,
a student must score a minimum of 50% of Practical mark and
subject to an overall average of 50%. Only students without
any re-sit or carry-over are eligible to sit for professional
examinations. The guiding policy also involves:
a) Attendance policies – 70% of lectures and laboratory
posting attendance is required
b) Laboratory posting
c) Appointment of External Examiners and MLSCN
Assessors
d) Council pass mark – 50%
e) Students should not carry any course over to any of the
professional examinations
f) A letter of intent and request for the appointment of
MLSCN Assessors must be written to MLSCN with
respect to the conduct of the professional examinations.
The First Professional Examination Consists of:-

12
Log Book Assessment (10marks)
MCQ: 20 practically oriented MCQs in each of Chemical
Pathology, Histopathology, Medical Microbiology,
Parasitology, Haematology and Blood Transfusion Science (30
Marks). Practical paper of 3hrs duration and including spot
tests (40marks). Oral examination in all disciplines (20marks).
Students who fail this examination shall be allowed to re-sit
after 3 months.
The Final Professional Examination consists of:-
Log book Assessment (10marks)
MCQ: 50 practically oriented MCQs in any of Chemical
Pathology, Histo-cytology, Medical Microbiology,
Parasitology, Haematology and Blood Transfusion Science (20
marks)
Practical Paper of 3hrs duration and including spot tests (50
marks)
Oral examination in one of the all disciplines (20 marks).
Students who fail this examination shall be allowed to re-sit
after 3 months.
Student project defence is part of the professional
examination.
Assessment of Professional Examination and Appointment
of External Examiners
For First Professional Examination (400L), University will
appoint an external examiner who should be a registered
member of Council and must not be below the rank of senior
lecturer. In addition, the Council will appoint an External
13
Assessor. For Final Professional Exam (500L), the university
will appoint an external examiner for each discipline, all of
which should be registered members of the Council and the
Council will also appoint an Independent Assessor.
INDUCTION OF GRADUATES
The induction of Medical Laboratory Science graduates is a
statutory function of the Medical Laboratory Science Council
of Nigeria. All graduates are inducted into the profession
following completion of their academic and professional
programmes. This involves administration of oath and
education on professionalism and ethics in their practice. The
induction should take place within 8 weeks of completion of
the degree examination.
Induction Guidelines
Compliance with the under listed requirements by the
Faculty/Department of Medical Laboratory Science is essential
before MLSCN will induct graduands of Medical Laboratory
Science (MLS) into the profession.
a) Presentation/submission of the approved final
examination result by the University Senate
b) Letter of intent and request for date from MLSCN with
a minimum of one month notice.
c) Induction Lecture should be presented by experienced
qualified Med Laboratory Scientist.
d) Sitting arrangements should be such that University
functionaries and Council officials are represented.
e) Order of procession – Inductees first, Lecturers,
University Functionaries, Registrar/CEO of Medical
Laboratory Science Council of Nigeria and the Vice
14
Chancellor. The reverse order is applied during
recession.
f) At induction, the inductees are presented with
provisional license, log book and Act Cap M25 LFN
2004.
INTERNSHIP PROGRAMME
In pursuance of Section 4(a) of Act 11 of 2003, Medical
Laboratory Science graduates are statutorily required to
undergo compulsory one-year continuous internship training
under the supervision of registered and licensed Medical
Laboratory Scientists in Medical Laboratory Science Council
of Nigeria approved internship centers (Hospital, Research
Institutes, and Medical Laboratories). Full registration which is
accompanied by issuance of license to practice as a Medical
Laboratory Scientist is granted after successful completion of
the internship programme.
DRESS CODE
MALE: A good pair of trousers (not jeans) with neat shirt, a
matching tie and a pair of shoes.
FEMALE: Corporate gown with sleeve or skirt (not jeans)
below the knee with sleeved shirt/blouse and a pair of shoes.
Student professional Lapel pin should be worn always on
their dresses/shirt
Wearing of Laboratory coat is compulsory for all clinical
laboratory postings and practical classes.

15
EXAMINATION MISCONDUCT IN
ACCORDANCE WITH THE UNIVERSITY
REGULATIONS
The following sanction shall apply to case of
examination misconduct as stipulated below.
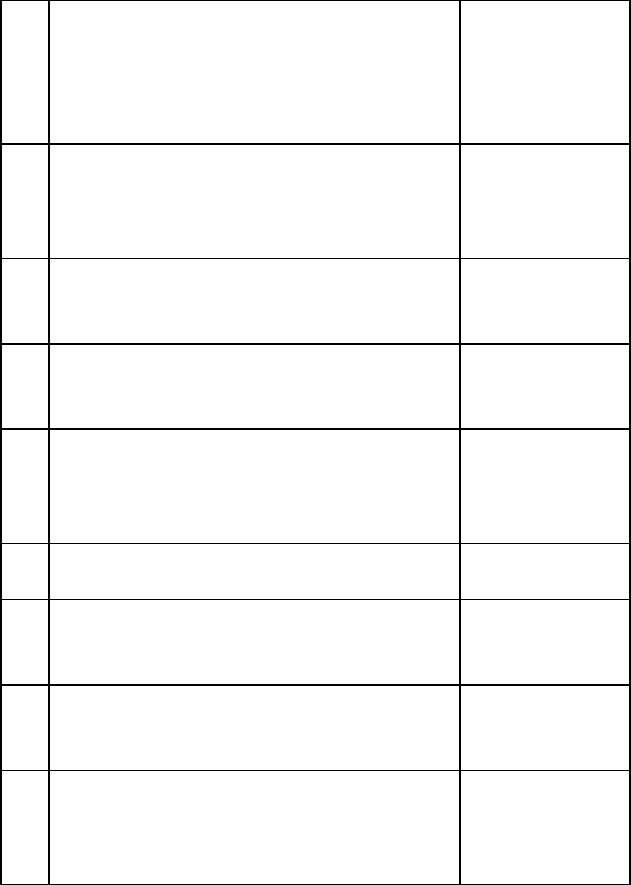
PENALTIES FOR EXAMINATION
MISCONDUCTS
S/N
Misconducts
Penalties
1.
Possession/copying of any written materials
relevant to the examination, tests and
assignments.
Rustication for
two semesters.
2.
Impersonation
Expulsion
3.
Plagiarism
Rustication for
one semester.
4.
Unauthorized access to examination
materials
Expulsion
5.
Unauthorized collection of items from
another student during an examination
without the knowledge of the invigilator
Letter of caution
6.
Falsification of evaluation form
and other academic records or documents
Expulsion
16
7.
Appearing for examination, without
meeting attendance requirement
Letter of caution
and prevention
from writing the
examination.
8.
Disobedience to instructions/ disruption
during an examination/harassment of
invigilator
Disqualification
from the
examination.
9.
Harassment of Invigilators
Rustication for
one semester.
10.
Anti-safety behaviour during practical,
workshops, studio work, etc.
Letter of caution
11.
Attempted inducement of examiners and
invigilators
Disqualification
from the
examination
12.
Aiding and abetting examination misconduct
Expulsion.
13.
Destruction of evidence of examination
misconduct
Rustication for
one semester
14.
Refusal to complete examination misconduct
form
Rustication for
one semester.
15.
Any previous arrangement made for access to
examination materials whether it succeeds or
not
Rustication for
two semesters.

17
GRADUATION REQUIREMENTS
Minimum number of the cumulative credit units as may be
approved from time to time and all core courses including the
professional examinations must be passed for a student to
graduate.
COURSE CODE SYSTEM
Course code contains an abbreviated letter code of three
(3) letters and three digits. MLS is a prefix that indicates
the department.
The first digit represents the level of study. For 100 – 500 levels,
the second digit number if odd denotes first semester and if
even it denotes second semester while the last digit denote
sequence
Core/Compulsory Course:
A course which every student must compulsorily take and pass
in any particular programme at a particular level of study.
Required Course
A course that you take at a level of study and must be passed
before graduation.
16.
Refusal to submit examination scripts
Failure in the
examined course.
17.
Any other misconduct recorded from time to
time
Penalty shall be
determined based
on the
recommendation
of the panel.
18
Elective Course
A course that students take within or outside the faculty.
Students may graduate without passing the course provided the
minimum credit unit for the course had been attained.
Optional Course
A course which students can take based on interest and may
count towards the minimum credit unit required for graduation.
Pre-requisite Course
A course which student must take and pass before taking a
particular course at a higher level.
Minimum Credit Load per Semester
The Minimum credit load per semester is 15.
Medical Laboratory Science Practicals
Practical contents of the Medical Laboratory Science courses are
taught under Laboratory postings and assessed using the format
of professional examination.
19
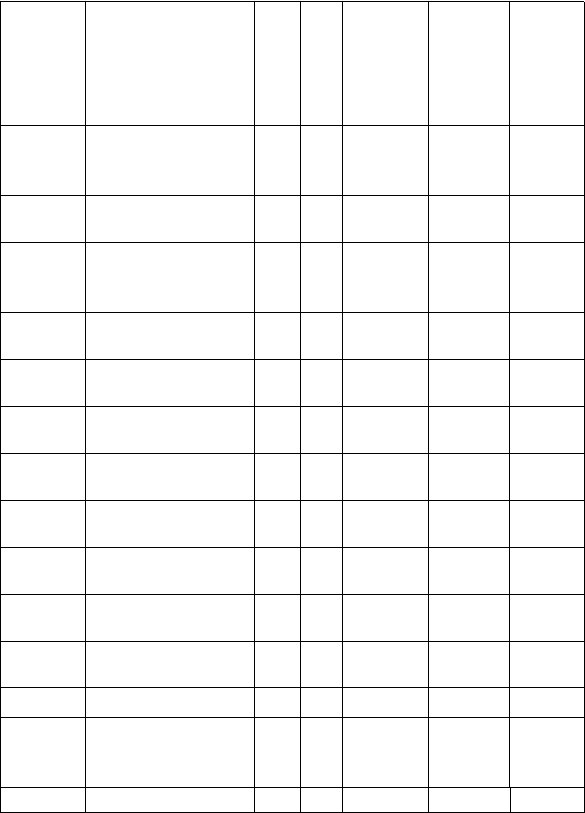
FIRST YEAR (100L) COURSES FOR THE DEPARTMENTOF MEDICAL
LABORATORYSCIENCE.
COURSE OUTLINE: 100 LEVEL MEDICAL LAB SCIENCE
FIRST SEMESTER
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
hour/ week
Practical
contact
hour/ week
Tutorial
Contact
hour/ week
MTH
111
Elementary
Mathematics I:
Algebra
3
C
3
0
1
CHM
111
General
Chemistry I
3
C
3
0
1
CHM
115
General
Chemistry
Practical I
1
C
0
1
0
PHY
111
General Physics
Practical I
3
C
3
0
1
PHY
119
General Physics I
1
C
0
1
0
GST
111
Communication
in English I
2
C
2
0
1
GST
112
Use of Library
and ICT
2
C
2
0
1
GST
114
Nigerian People
& Culture
2
R
2
0
1
BIO
111
General Biology
3
C
3
0
1
BIO
117
General Biology
Practical I
1
C
0
3
0
CSC
111
Introduction to
Computer Science
2
C
2
0
0
CIT III
IT Certification
0
R
2
0
0
EDS
111
Entrepreneurial
Development
Studides
1
C
1
3
0
TOTAL
24
23

20
SECOND SEMESTER
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
hour/week
Practical
contact
hour/week
Tutorial
Contact
hour/week
BIO 121
General Biology II
3
C
3
0
1
BIO 127
General Practical Biology II
1
C
0
3
0
PHY 121
General Physics II
3
C
3
0
0
PHY 129
Experimental Physics II
1
C
0
1
0
CHM 121
General Chemistry II
3
C
3
0
0
CHM 129
Introductory Chemistry II
3
C
3
0
0
CSC 121
Introduction to Computer
Science
2
C
2
0
1
GST 121
Communication in English II
2
C
2
0
1
CIT 121
IT Certification I: MS Excel
0
R
1
1
0
EDS 121
Entrepreneurship Dev.
Studies II
1
R
1
1
0
MTH 121
General Mathematics II
3
C
2
0
1
BTG
121
Introduction to
Biotechnology
1
R
1
0
0
TOTAL
23
23
SECOND YEAR (200L) COURSES
FIRST SEMESTER
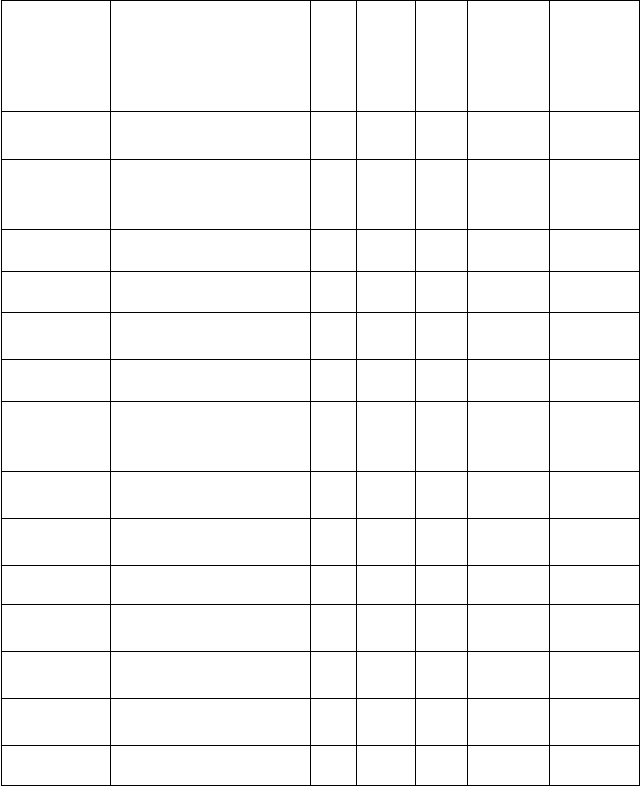
21
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
hour/
week
Practical
contact
hour/wee
k
Tutorial
Contact
hour/wee
k
ANA 211
Human Anatomy
2
C
2
I
0
MLS 211
Introduction to
Medical Laboratory
Science I
2
C
2
0
1
PIO 211
Human Physiology
2
C
2
3
0
PHS 219
Practical Physiology I
0
R
3
3
0
ANA 212
History of Basic
Tissues
2
R
2
1
ANA 219
Anatomy Practical
1
R
0
3
0
MLS 212
Medical
Microbiology &
Parasitology
2
C
2
2
1
PIO 212
Principle of Cell
Physiology I
2
R
2
2
0
GST 113
Logic, Philosophy of
Human Existence
2
R
2
0
0
STA 111
Medical Biostatistics
2
R
2
0
1
BCH 211
General Medical
Biochemistry
3
R
3
0
1
BCH 219
Practical
Biochemistry I
1
R
0
3
1
GST 213
Peace Conflict &
Resolution Studies
2
R
2
0
0
TOTAL
24
24
SECOND SEMESTER
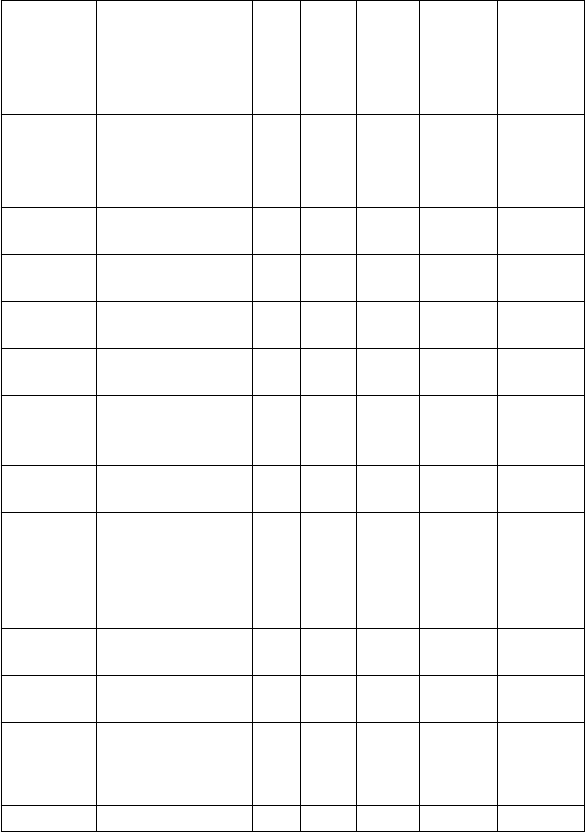
22
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
Hour/Wee
k
Practical
Contact
Hour/Wee
k
Tutorial
Contact
Hour/Wee
k
MLS
221
Introduction to
Medical
Laboratory
Science II
2
C
2
0
1
ANA
221
Gross Anatomy
2
C
2
1
0
ANA
222
Systemic
Histology
2
C
2
1
0
BCH
221
Introductory
Biochemistry II
3
C
3
0
0
ANA
224
General
Embryology
2
C
2
0
1
ANA
229
Human
Anatomy
Practicum II
2
C
0
4
0
BCH
223
Bioenergetics
3
C
3
3
0
PIO 221
Autonomic
Nervous System
Gastro Intestinal
Tract and Renal
Physiology
3
C
3
3
0
PIO 222
Endocrinology
& Reproduction
3
C
3
3
0
GST 222
Leadership
Skills
2
R
2
0
1
BCH
229
Practical
Biochemistry II
1
R
0
3
0
TOTAL
24
24
THIRD YEAR (300L) COURSES
FIRST SEMESTER

23
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
Hour/
Week
Practical
Contact
Hour/
Week
Tutorial
Contact
Hour/Wee
k
MLS 316
Laboratory
Posting I and
Practical
3
C
0
9
0
MLS 312
Basic
Haematology
3
C
3
3
0
MLS 314
Basic
Histopathology
3
C
3
0
0
MLS 311
Basic Clinical
Chemistry
3
C
3
3
0
BCH 311
Chemistry &
Metabolism of
Amino Acids &
Proteins
2
R
2
0
0
MLS 313
Basic Medical
Microbiology
2
C
2
2
2
MLS 315
Basic
Immunology
2
R
2
0
0
EDS 311
Entrepreneurship
1
R
1
0
1
MLS 315
Biostatistics
2
C
2
0
1
PHE 222
The Principles
of
Epidemiology
and Disease
Surveillance
1
R
1
0
0
BCH 319
Practical
Biochemisty III
1
C
0
3
0
TOTAL
23
23
300 LEVEL - SECOND SEMESTER
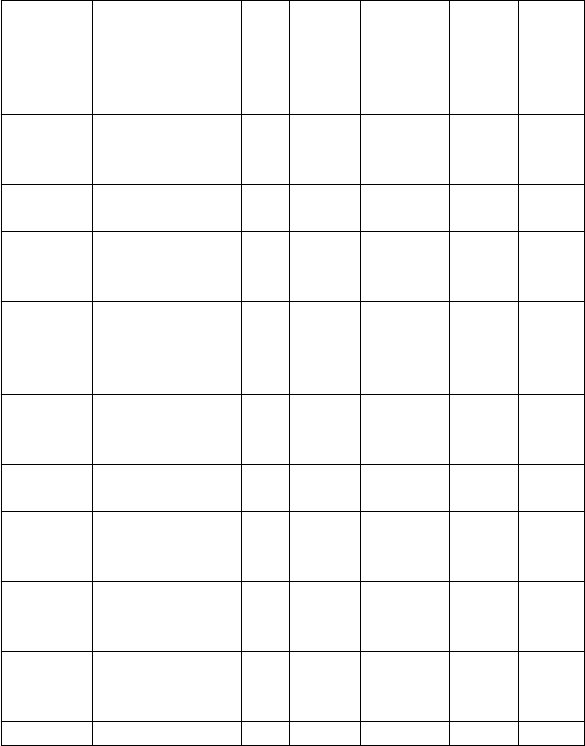
24
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
Hour/Wee
k
Practical
Contact
Hour/
Week
Tutorial
Contact
Hour/
Week
MLS
321
Laboratory
Posting and
Practicals II
3
C
0
9
0
MLS
322
Basic
Parasitology
3
C
3
3
0
MLS
323
Laboratory
Instrumentation
& Techniques
3
C
3
3
0
MLS
324
Fundamental
Blood
Transfusion
Science
2
C
2
2
0
MLS
325
Medical
Laboratory
Science Ethics
2
C
2
0
0
MLS
326
Basic
Histopathology
3
C
3
3
0
MLS
327
Laboratory
Management &
Organization
2
C
2
0
0
PCO
321
Basic
Pharmacology
and toxicology
2
C
2
0
0
PCO322
Practical
Pharmacology
and toxicology
1
C
0
3
0
TOTAL
21
21
N/B:Year three marks the beginning of the professional
training. Core Courses are handled by qualified and registered
Medical Laboratory ScientistsMLS 311 and 321 are assessed as
Oral, MCQ, Practicals and Logbook/Hospital Laboratory
posting performance.
400 LEVEL - FIRST SEMESTER

25
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
Hour/Wee
k
Practical
Contact
Hour/Wee
k
Tutorial
Contact
Hour/Wee
k
MLS 411
Laboratory
Posting III
and Practicals
3
C
‘0
9
0
MLS 412
Medical
Parasitology
& Entomology
3
C
3
3
0
MLS 413
Basic Medical
Bacteriology
& Mycology
3
C
3
3
0
MLS 414
Introduction to
Blood Group
Systems,
Compatibility
Tests
Hemoglobin
and
haemoglobino
pathy
3
C
3
3
0
MLS 415
Analytical
Clinical
Chemistry
3
C
3
3
0
MLS 416
Nucleic Acid
Biochemistry
& Basic
Concepts of
Molecular
Biology
2
C
2
0
0
MLS 417
Cytological
techniques
1
C
1
3
0
TOTAL
18
18
400 LEVEL - SECOND SEMESTER

26
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
Hour/Week
Practical
Contact
Hour/Week
Tutorial
Contact
Hour/Week
MLS
421
Laboratory Posting
IV and Practicals
3
C
0
9
0
MLS
422
Virology
3
C
3
3
0
MLS
423
Histopathology and
Museum
Techniques
3
C
3
3
0
MLS
424
Biomedical
Engineering
2
C
3
3
0
MLS
425
Biotechnology &
Bioinformatics
2
C
2
0
0
MLS
426
Counseling skills
2
C
2
0
1
MLS
427
Immunology/Immu
nochemistry
3
C
3
0
0
MLS
428
First professional
examination
3
C
0
9
0
PHA
421
Chemotherapy of
Microbial
Diseases,Vaccines
and Sera
2
R
2
0
0
TOTAL
23
23
MLS 411, 421 and 428 are assessed as Oral, MCQ, Practicals
and Logbook/Hospital Laboratory posting performance.
FIFTH YEAR (500L) COURSES

27
CHEMICAL PATHOLOGY SPECIALTY
FIRST SEMESTER
COURSE CODE
COURSE TITLE
UNIT
STATUS
Lecture Contact
Hour/ Week
Practical Contact
Hour/ Week
Tutorial Contact
Hour/ Week
MLS 511
Laboratory
Posting V and
Practicals
3
UOG
C
0
9
0
MLS 512
Seminar
2
C
0
0
2
MLS 513
Research
Methodology
3
C
2
0
1
MLS 514
Carbohydrate,
protein and Lipid
Metabolism
3
C
3
3
0
MLS 515
Renal, Liver and
Neurochemistry
3
C
3
3
0
MLS 516
Clinical
Enzymology
3
C
3
3
0
MLS 517
Nutrition and
Clinical
Vitaminology
2
C
2
0
0
TOTAL
19
19
SECOND SEMESTER

28
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
Hour/ Week
Practical
Contact
Hour/ Week
Tutorial
Contact
Hour/ Week
MLS 521
Laboratory Posting VI
and Practicals
3
C
0
9
0
MLS 522
Genetics & Molecular
Biology
2
C
2
0
0
MLS 524
Project
6
C
0
6
0
MLS 526
Drug Monitoring,
Toxicology & Inborn
Error of Metabolism
3
C
3
0
1
MLS 527
Clinical &
Reproductive
Endocrinology
3
C
3
3
0
MLS 528
Techniques in Clinical
Chemistry
3
C
3
3
0
MLS 540
Final Professional
examination
3
C
0
9
0
TOTAL
23
23

29
HAEMATOLOGY & BLOOD TRANSFUSION SCIENCE
(SPECIALTY)FIRST SEMESTER
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact
Hour/Week
Practical
Contact
Hour/Week
Tutorial
Contact
Hour/Week
MLS 511
Laboratory Posting V
and practicals
3
C
0
9
0
MLS 512
Seminar
2
C
0
0
2
MLS 513
Research
Methodology
3
C
3
0
1
MLS 519
Cytogenetics
2
C
2
0
0
MLS 531
Haemopoiesis,
Hemoglobin,
Haemoglobinopathies
&Myeloproliferations
3
C
3
3
0
MLS 532
Blood Group Systems
& Compatibility
Tests
3
C
3
3
0
MLS 533
Serology & Blood
Transfusion Science
3
C
3
3
0
TOTAL
19
19
SECOND SEMESTER

30
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture Contact
Hour/ Week
Practical
Contact Hour/
Week
Tutorial
Contact Hour/
Week
MLS 521
Laboratory Posting VI
3
C
0
9
0
MLS 522
Genetics & Molecular
Biology
2
C
2
0
0
MLS 524
Project
6
C
0
6
0
MLS 542
Advanced Haematological
Techniques
3
C
3
3
0
MLS 543
Advanced Blood Group
Serology Techniques
3
C
3
3
0
MLS 544
Coagulation and Fibrinolysis
Studies
3
C
3
3
0
MLS 540
Final Professional
examination
3
C
0
9
0
TOTAL
23
23
HISTHOPATHOLOGY (SPECIALITY)
FIRST SEMESTER

31
COURSE CODE
COURSE TITLE
UNIT
STATUS
Lecture Contact
Hour/ Week
Practical Contact
Hour/ Week
Tutorial Contact
Hour/Week
MLS
511
Laboratory
Posting V and
practicals
3
C
0
9
0
MLS
512
Seminar
2
C
0
0
2
MLS
513
Research
Methodology
3
C
3
0
1
MLS
519
Cytogenetics
2
C
2
0
0
MLS
534
Fundamental
Histopathology
3
C
3
3
0
MLS
535
Systemic
Histopathology
3
C
3
3
0
MLS
536
Histochemistry
and
Histopathological
Techniques
3
C
3
3
0
TOTAL
19
19

32
SECOND SEMESTER
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture Contact
Hour/Week
Practical
Contact
Hour/Week
Tutorial
Contact
Hour/Week
MLS 521
Laboratory
Posting VI
3
C
0
9
0
MLS 522
Genetics &
Molecular
Biology
2
C
2
0
1
MLS 524
Project
6
C
0
6
0
MLS 548
Medical
Cytology
2
C
2
0
0
MLS 561
Embalmment
Science and
Museum
Techniques
2
C
2
2
0
MLS 562
Immunochemi
stry
2
C
2
2
0
MLS 563
Stains and
Staining
Techniques
3
C
3
3
0
MLS 540
Final
Professional
examination
3
C
0
9
0
TOTAL
23
23

33
MEDICAL MICROBIOLOGY (SPECIALITY)
FIRST SEMESTER
COURSE
CODE
COURSE
TITLE
UNIT
STATUS
Lecture
Contact Hour/
Week
Practical
Contact Hour/
Week
Tutorial
Contact Hour/
Week
MLS 511
Laboratory
Posting V
and practical
3
C
0
9
0
MLS 512
Seminar
2
C
0
0
2
MLS 513
Research
Methodolog
y
3
C
3
0
1
MLS 537
Systematic
Bacteriology
3
C
3
3
0
MLS 538
Advanced
Entomology
2
C
2
0
1
MLS 539
Public
Health
Microbiolog
y
3
C
3
3
0
MLS 551
Medical
Mycology
3
C
3
3
0
TOTAL
19
19
SECOND SEMESTER

34
COURSE
CODE
COURSE
TITLE
CREDIT
LOAD
STATUS
Lecture
Contact
Hour/ Week
Practical
Contact
Hour/ Week
Tutorial
Contact
Hour/Week
MLS 521
Laboratory Posting
VI
3
C
0
9
0
MLS 522
Genetics &
Molecular Biology
2
C
2
0
1
MLS 524
Project
6
C
0
6
0
MLS 564
Medical Virology
3
C
3
3
0
MLS 566
Pharmaceutical
Microbiology &
Microbial Genetics
3
C
3
3
0
MLS 568
Laboratory
Techniques in
Microbiology
3
C
3
3
0
MLS 540
Final Professional
examination
3
C
0
9
0
TOTAL
23
23
DESCRIPTION OF COURSES
200 LEVEL COURSES
FIRST SEMESTER COURSES
35
MLS 211: INTRODUCTION TO MEDICAL
LABORTORY SCIENCE I (2 Units)
General introduction to medical laboratory science subjects
namely, Clinical Chemistry, Haematology and Blood
Transfusion Science. Medical Microbiology, Histopathology
and Immunology, Specimen collection, reception and
registration. Storage and disposal, Specimen bottle. Safety
precaution in pathology laboratories against chemical,
biological, electrical materials and radiation hazards.
Techniques and principles of chemical sterilization and
physical methods. Glassware cleaning care and maintenance,
Breeding of laboratory animals.
ANA 211: HUMAN ANATOMY, ANATOMY OF UPPER
LIMBS ANDLOWER LIMBS(3 units)
Descriptive terms, plans and terms of relationship of the human
body, terms of comparison, attachment of muscles, types of
muscles, movements of joints. Osteology, Principles of
Kinesiology, general organization of body systems. Osteology
of the upper limb, pectoral region and the breast, brachial
plexus, scapular region and the axilla, shoulder joint, arm,
cubital fossa and elbow. Forearm, wrist joint and hand. Vessels
and lymphatic drainage of the upper limb. Surface anatomy,
applied and radiological anatomy of the upper limb. Osteology
of Lower Limb, front of the thigh I (femoral triangles,
femoral canal and hernia, sub-sartorial canal). Front of thigh II,
medial side of the thigh, gluteal region, back of the thigh,
popliteal fossa, front of the leg and the dorsum of the foot,
lateral side of the leg, back of the leg, sole of the foot (aches of
the foot). Hip joint and the knee joint, tibio-fibular joints and
ankle joints. Surface anatomy, applied and radiological
anatomy of the lower limb.
ANA 212: HISTOLOGY OF BASIC TISSUES (3Units)
Description: Structure and the function of the cell, general
histology and basic tissues of the body. Preparation of tissues
36
for microscopy is a practical oriented course that is studied
alongside with the theoretically based lecture.
ANA 224: GENERAL EMBRYOLOGY (3 Units)
General consideration of the male and female reproductive
organs. Gametogenesis, fertilization, implantation, cleavage,
the morula, the blastocyst formation of the primitive streak, the
Bilaminar and trilaminar germ disc. Development of tissues
and organ systems of the embryo, the chorionic and amniotic
cavities. Foetal membranes, placental formation and functions.
The molecular regulation in differentiation of tissues and
organs and in the establishment and patterning of the body axis.
Birth defects, chromosomal and genetic factors. Twins and
twin defects, general characteristics of the embryonic
environment and foetal periods.
BCH 211: GENERAL AND MEDICAL BIOCHEMISTRY
(3 Units)
Short history and Definition of Biochemistry. Importance of
Biochemistry to medicine and other scientific disciplines. The
living cell. Organization and Molecular architecture. Types of
cells and their characteristics. Structure and organization of
Biological membranes. Biomolecules and the origin of life.
Chemistry of Biomolecules. Carbohydrates Classification,
structure, distribution and functional role of named examples.
Chemical properties and reactions. Essential fatty acids,
Eicosanoids, fat-soluble vitamins – Structure and functions.
Peptide bonds and hierarchy of protein structure Nucleic acids.
RNA and DNA. Structure/function of enzymes. Zymogens.
Active site and specificity of enzymes. Inhibition and
Activation of enzymes. Factors affecting enzyme catalysis
reaction. Allosteric enzymes. Isoenzymes. The Concepts of
Avitaminoses, Hypovitaaminoses and Antivitamins. Vitamins
and their Co-enzyme function. Biomedical importance of
vitamins.
37
BCH 211 EXPERIMENTAL/ PRACTICAL
BIOCHEMISTRY (2 Unit)
Laboratory exercises on the practical contents of introductory
biochemistry
PIO 211: HUMAN PHYSIOLOGY (3Units)
Cell physiology, Physiochemical principles, Body fluids and
Blood transport: Control systems. Excitable and contractile
Cells. Introduction to ANS. Introduction and definition of body
fluids and body fluid compartments. Regulation of body fluid
volumes. Physiological variation of body fluid volumes.
Techniques for quantifying various body fluid volumes. Blood:
Functions of blood and classifications of blood cells.
Erythropoiesis.Haematologicalindices.Hemoglobin genotype
and Blood groups. Immunology and cell defence
PIO 212: PRINCIPLES OF CELL PHYSIOLOGY (3 Units)
Definition and functions of the cardiovascular system, Cardiac
muscle, Cardiac myoelectrophysiology, cardiac cycle,
Circulation of blood: cardiac output and regulation. Blood
pressure.Haemodynamics and microcirculation.Pulmonary,
Cerebral, Coronary, Splanchnic and muscle circulation, Shock
and cardiovascular changes in exercise.Definition and
functions of the respiratory system, Physiologic anatomy of the
respiratory system.Respiratory dynamics and work. Pulmonary
ventilation: Lung volumes and capacities, Spirometry.
Mechanism and mechanics of breathing, Lung surfactant,
pulmonary circulation. Gas exchange and Gas transport.
Oxygen Haemoglobin dissociation curve.Hypoxia and
Dyspnea.Respiratory changes in exercise and barometric
changes.Control of breathing
GST 221: CONTEMPORARY HEALTH ISSUES (2 Units)
38
Diet, exercise and health, nutritional deficiency diseases,
malaria, other infections, hypertension, organ failure, air-borne
diseases, sexually transmitted diseases, cancer and its
prevention, sickle cell disease. HIV/AIDS: Introduction,
epidemiology of HIV, natural history of HIV infection,
transmission of predisposing factors to HIV, Impact of
HIV/AIDS on the society, management of HIV infection,
prevention of HIV. Drugs and Society: sources of drugs,
classification of drugs, dosage forms and routes of drug
administration, adverse drug reactions, drug abuse and misuse,
rational drug use and irrational drug use. Human kinetics and
health education: personal care and appearance, exercise and
health, personality and relationship, health emotions, stress,
mood modifiers, refusal to tobacco, alcohol and other
psychoactive drugs.
ENT 201: INTRODUCTION TO ENTREPRENEURIAL
STUDIES (2 Units)
This course introduces students to the definition, functions,
types, and characteristics of entrepreneurship. This course
further examines entrepreneurship and ethics, entrepreneurship
theories and practice; new venture creation; forms of business,
business opportunities, starting a new business, innovation,
legal issues in business, insurance, and environmental
considerations, possible business opportunities in Nigeria and
introduction to biographies of successful entrepreneurs etc.
MLS 221: INTRODUCTION TO MEDICAL
LABORATORY SCIENCES II (2 Units)
Microcopy and micrometry-use and care of microscopes.
Refrigeration and freeze-dries-principles, uses, care and
maintenance. Handling of laboratory animals. Laboratory
location and floor plan. Laboratory organization and
management. Simple analytical techniques in chemical
pathology. Presentation of volumetric analysis. Urinalysis,
39
principles of tissues preservation; fixation, processing and
staining. Handling of surgical autopsy specimens. Removal of
formal in pigments, basic, tools of the microbiologist-wire loop
cotton wool, pipettes, swab and their uses, preparation of films
and basic staining techniques: Gram’s stain, ZiehlNeelson’s
stain. Hematological stain principle and components. Blood
film preparation and staining, pipettes, chambers care and uses.
Haemoglobin, PCV estimation, WBC counting. Evolution of
Medical laboratory science in Nigeria. History of medical
laboratory Laboratory practices and training. Regulatory body.
Contributions of IBMS. MLSCN Act, 2003.
ANA 221: GROSS ANATOMY (3 Units)
Thorax - Osteology of the thoracic cage, intercostal space
(intercostal muscles, vessels and nerves), pleura and lungs,
mediastinum, cardiac plexus and thoracic diaphragm.
Abdominal alimentary tract, liver, spleen, pancreas and kidneys
and suprarenal glands Pelvis and Perineum: Male and female
perineum, pelvic wall and floor, pelvic peritoneum, viscera,
nerves and vessels. Surface anatomy, Radiological anatomy.
ANA 222: SYSTEMIC HISTOLOGY (3 Units)
Cardiovascular system, skin, glands of the skin, Structure of
nails and hair. Respiratory system. Digestive system. Urinary
and genital system. Electron micrograph studies of each organ
BCH 221:INTRODUCTORY BIOCHEMISTRY II (3 units)
Structural inter-relationships of sugars. Stereochemistry of
sugars. Hexoses, pentose’s, disaccharides, starch, glycogen and
polysaccharides. Methods of identifying sugars. Carbohydrate
metabolism, digestion and absorption. Control of glycolysis,
TCA cycle and pentose phosphate pathway. Glyoxylate
pathway, gluconeogenesis, glycogenolysis, glycogenesis.
Mitochondrial electron transport chain and oxidative
40
phosphorylation. Energy generation and storage in biological
systems. Disorders of carbohydrate metabolism. The pyruvate
and alpha-ketoglutarate complexes and their regulation.
Metabolism of lipids, digestion, absorption. Role of
lipoproteins in lipid transport. Metabolism of lipoprotein in
health and disease. Triacylglycerol oxidation and oxidation of
fatty acids. Storage and mobilization of energy stores in
adipocytes. Ketone bodies and ketosis. Interrelationships of
fatty acid and carbohydrate biosynthesis/ oxidation. Biological
importance of eicosanoids, glycolipids and sphingolipids. The
chemistry and metabolism of steroids and steroid hormones.
Biochemical rearrangement in G-6-PD deficiency. Sickle cell
anemia. Glycogen storage disease etc. Illustrative laboratory
exercises.
BCH 223: BIOENERGETICS (2 Units)
An outline of biologic oxidations leading to intermediary
metabolism of carbohydrates, lipids, proteins, amino acids,
nucleic acids and nucleotides. Electron transport and oxidative
phosphorylation’s ATP and other high energy compounds and
their importance.
PIO 221: AUTONOMOUS NERVOUS SYSTEM,
GASTRO- INTESTINAL TRACT AND RENAL
PHYSIOLOGY (3 Units)
Definition and functions of the kidney.Physiologic anatomy of
the kidney.Glomerular filtration. Tubular functions. Urine
formation: - Dilute and concentrated urine. Counter current
mechanism, Plasma clearance, renal autoregulation, ECF
regulation, Acid-Base balance, Renin-Angiotensin system.
Physiology of excitable tissues. Functional organization of
Autonomic Nervous System, basic characteristics of
41
sympathetic and parasympathetic divisions. Introduction to
human Genetics, Biotechnology and Human Genome.
Definition and functions, Physiologic anatomy and
Innervations of the GIT, Mastication, Deglutition, Salivary
gland, Digestion and food absorption, Movement and Stomach
emptying, Movements of the GIT, Vomiting and defecation,
GIT secretions and juices, Liver and General metabolism
(BMR).
PIO 222: ENDOCRINOLOGY & REPRODUCTION(3
Credits)
Definition and functions, Definition of Hormones, Methods of
Measurement, Types and mechanism of Actions, Regulation,
Physiologic anatomy, Hypothalamus-Hypothalamic releasing
factors, Hypothalamic Nuclei, Hypothalamo-hypophyseal
system, Pituitary gland, Tropic Hormones, GIT and other local
hormones. Structure and functions of male and female
reproductive organs, Androgens, Spermatogenesis and
fertility.Infertility in male.Monogenesis, Sexual cycle and
hormonal regulations.Fertilization, Pregnancy and Parturition,
Fertility and infertility in female.Family planning.
GST 222: LEADERSHIP SKILLS (2 Units)
Transformation is a fundamental shift in the deep orientation of
a person, organization or society such that the world is seen in
new ways and new actions and results become possible that
were impossible prior to the transformation. Transformation
happens at the individual level but must be embedded in
collective practices and norms for the transformation to be
sustained. Leadership development programme (LDP) proposes
novel approaches to teaching and learning, which emphasizes
42
the practical involvement of participants. It is interactive and
involves exercises and actual implementation of breakthrough
projects by teams that make difference in the lives of the target
population. In this course, leadership concepts comprising of
listening, conversation, emotional intelligence, breakthrough
initiatives, gender and leadership, coaching and leadership,
enrolment conversation and forming and leading teams will be
taught.
GST 213: PEACE STUDIES AND CONCLICT RESOLUTION (2
Units)
This course provides the basic concepts in peace studies and
conflict resolution, peace as vehicle of unity and development,
conflict issues, types of conflicts, e.g. ethnic, religious, political,
economic conflicts, root causes of conflicts and violence in
Africa, indigene, settler phenomenon, peace building,
management of conflict and security,elements of peace studies
and conflict resolution, developing a culture of peace, peace
mediation and peace-keeping, alternative dispute resolution
(ADR), dialogue/arbitration in conflict resolution, Role of
international organizations in conflict resolution, e.g.
ECOWAS, African Union, United Nations, etc.
PHE 222: The Principles of Epidemiology and Disease
Surveillance (3 Units)
This is an introductory course designed to acquaint the
student with the basic principles of epidemiology. It is
intended for the undergraduates in the medical, nursing,
public health, medical laboratory sciences and other
health-related programs. Attention is focused on the
historical context and developments, definition of terms
43
and concepts, scope, uses, concepts of disease causation,
measures of disease frequency, levels of prevention, types
and methods of epidemiological investigations. Students
for demonstration and illustration use available medical
information and statistics as laboratory materials. The
approach is to provide opportunity for students to become
acquainted with the basic principles of epidemiology
which are important tools in Public Health Sciences
.
300 LEVEL COURSES:
BCH 321: CHEMISTRY AND METABOLISM OF
AMINO ACIDS AND PROTEIN (3 Units)
Amino acids as building blocks of proteins, amino acid
sequence of proteins, covalent backbone of proteins.
Chemistry/structure/Reactions/classification of amino acids,
Properties of the peptide bond. Levels of organization of
proteins. Protein isolation, fractionation, purification and
characterization. Biological functions of proteins. Genome
organization. Evidence for DNA as the carrier of genetic
information. Purines and pyrimidines, Nucleoside and
Nucleotide Structure and Nomenclature, abnormalities in
nucleic acid metabolism, Structure of RNA & DNA.DNA
Organization into Chromosomes. Early foundation for DNA
structures, Forces involved in DNA helices, Denaturing and
Annealing, Hypochromic Effect, Erwin Chargaff`s rule.
Metabolism of one carbon units, metabolism of inorganic
nitrogen
EDS 311: ENTREPRENEURIALSKILLS ((2 Units)
The course focuses the attention of the students to the practical
aspects of entrepreneurship by venturing into any of the
44
following categories: agriculture/agro allied, (fish farming, crop
production, animal husbandry such as poultry, piggery, goat etc.
groundnut oil making, horticulture (vegetable garden, flower
garden); services (bakery, radio/TV repairs, barbing/hair
dressing salon, car wash, catering, courier, event planning,
fashion design, vehicle maintenance, film production, interior
decoration, laundry, music production, phone call center, rental,
restaurant, tailoring/knitting, viewing center); manufacturing
(carving, weaving, sanitary wares, furniture making, shoe
making, plastic making, table making, bead making, bag
making, sachet water production, cosmetics, detergents);
commerce (buying and selling, purchasing and supply,
bookkeeping, import and export etc.); information and
communication technology (ICT) (business center, computer
maintenance, handsets repairs, internet café etc.);
mining/extraction (kaolin, coal mining, mental craft such as
blacksmith, tinsmith, etc., vegetable oil/and salt extractions
etc.); environment (fumigation, household cleaning waste
disposal etc.); tourism (car hire, craft work, hotel/catering,
recreation center); power (generator mechanic, refrigeration/air
conditioning, electricity wiring etc.); production/processing
(glassware production/ceramic, metal work/fabrication, steel
and aluminum door and windows, paper production, water
treatment/conditioning/packaging, bricklaying, iron welding,
building drawing, tailoring, carpentry, leather tanning, printing,
food processing/packaging/preservation). Students are to select
two of the above areas of interest for practical. Topics should
also include products/service exhibition and quality control,
business ownership structures, mentorship.
MLS 316: LABORATORY POSTING AND PRACTICAL
I (3 Units)
45
Posting of students to all sections of routine medical
laboratories for on the job training under the supervision of
qualified medical laboratory scientists for 2 days weekly for the
entire semester. Scored log books are kept by each student per
posting.
MLS 325: MEDICAL LABORATORY SCIENCE ETHICS
(3 Units)
History and philosophy of ethics in the practice of Medical
Laboratory Science. Relationship between religion and socio—
cultural values on medical ethics. Ethical issues involved in
private practice. Relationship between the Medical laboratory
scientist and other members of the health team.Intra
professional auditing, Medicallaboratory Sciences ethic and
consultancy services. Elements of informed consent in research.
Relationship between proper dressing, personal comportment
and patient care-the psychologist’s view: Medical Laboratory
Science ethics as it affects paternity disputes, infertility studies,
and sexually transmitted disease etc. real case presentation
medico—legal aspects of medical Laboratory practice
MLS 322: BASIC PARASITOLOGY(3 Units)
Introduction to parasitism, and other animal associations,
adaptation to parasitic way of life. How parasites invade their
host. The ineffective agents of parasites.Basic knowledge of
structure, classification and life cycle of parasites of medical
importance, vectors and intermediate hosts of parasites.
Introduction to anthropoids of medical importance. Biology of
the mosquito in relation to the transmission of malaria,
filariasis and viral infections.
MLS 326: MEDICAL PHYSICS(3 Units)
Kinematical and mathematical problems—circulation of pulse,
blood pressure and volume changes. The heart and blood
surface tension effect. Temperature and heat flow/electricity,
electrocardiograms, general radiation linear energy transfer and
46
radiation measurement, radiation damage-detection and safety,
X-ray generation and application radioisotopes production, use
and disposal.
MLS 311: BASIC CLINICAL CHEMISTRY (3 Units)
Traditional and S. 1 units in clinical chemistry; Reference
values: Gastric function test; Agents for Gastric stimulation.
Ward procedures and laboratory investigation of Gastric
secretion: Intestinal function tests; Digestion and absorption;
cause of Malabsorption. Laboratory investigation of
malabsorption. Renal functions of the kidney; measurement of
Renal plasma flow, Glomerular filtration rate. Creatinine
clearance, insulin clearance, concentration and dilution tests;
urinary, acidification tests, urine specific gravity/Osmolality.
Dye Excretion test. Water and Electrolyte status. Blood buffers.
Transport of blood gases; assessment of acid/base status. Lipids;
definition and types of lipids; formation of free fatty acids,
Ketone bodies and lactate; measurement of plasma lipids and
lipoprotein. Plasma proteins and physiology functions; factors
affecting synthesis and catabolism. Methods for the
determining of total protein in serum. Carbohydrate
metabolism; blood glucose homeostasis, Hyperglycemia
diabetes mellitus-its cause and investigation: Hypoglycemia—
types causes and investigation.
MLS 315: BASIC IMMUNOLOGY (2 Units)
The Historical background of Immunology. Classification of
Immunity Innate Immunity. Development and structure of cells
in the Immune system. Cellular interaction in the expression
and regulation of immunity acquired.
MLS 317: BIOSTATISTICS (3 Units)
Aims, characteristics and application of biostatistics in
biomedical sciences- samples, population variables, frequently
distribution, vital and descriptive statistics, measurement of
central tendencies – mean, median, mode dispersion, standard
47
deviation and coefficient of variation. Collection and
presentation of data, probability distribution. Hypothetical tests
of statistical significance. Analysis of variance, regression and
correlation, experimental designs and clinical trails.
MLS 321: LABORATORY POSTING AND PRACTICAL
II (3 Units)
Posting of students to all section of routing medical
laboratories for on-the-job training under the supervision of
qualified medical laboratory scientist for 2 days per week
scored logbook records per bench are kept for each student per
posting.
MLS 313: BASIC MEDICAL MICROBIOLOGY(2 Units)
History, Morphology, growth and nutrition. Classification and
identification of bacteria. Bacterial genetics, Bacteriophages,
viruses, infection and resistance to infection. Sterilization and
disinfection. Antimicrobial agents. Introduction to parasites
and fungi.
MLS 323: MEDICAL LABORATORY
INSTRUMENTATION &TECHNIQUES (3 Units)
Instrument aspects of qualitative and quantitative analysis -
theory and practice of some common analytical techniques;
colorimetry, spectro-flourimetry flame - photometry,
conductometry, polarography etc. Osmometry, nephelometry,
Turbidometry, PH measurement by ion specific electrodes—
separation techniques including Electrophoresis; paper,
cellulose acetate, Agar gel starch and polyacrylamide gel;
Isoelectric focusing, Isotophoresis, Chromatography, Ion
exchange, Gel filtration, molecular sieves; dialysis filtration,
solvent Extraction, centrifugation – Ultracentifiguration,
Immunoeletrophoretic techniques, radio immunoassay,
48
competitive protein binding, Isotope dilution techniques;
Enzyme Immuno assay, receptor Assay, automation, Micro and
Ultra Micro Analysis. Practical based on the above topics.
MLS 324: FUNDAMENTAL BLOOD TRANSFUSION
SCIENCE (2 Units)
ABO and Rhesus Blood Groups, Inheritance, distribution and
Genetic Theory. Blood Grouping Techniques –principles,
Disadvantages and Advantages. Preparation of Antisera,
Antiserum titration avidity, potency and specificity. Plant
lectins—preparation and standardization of antisera from
lectinase.Dolochosbiflorus. Anticoagulants used in BGS; ACD,
CPD-CPA-A etc in modes of action and side effects. Blood
bottles (MRC) and plastic Bags-Advantages and disadvantages.
Donor screening—using CUSO4 method –other methods of
screening. Preparation of blood products—cryoprecipitate,
platelet rich plasma, packed cells fresh frozen plasma,
fibrinogen etc. storage of blood and blood products- various
methods, advantages and disadvantages. Blood banking –
organization structures, facilities and records. Blood group
specific substance – synthesis, identification method (s) and
application. Quality control of physical and chemical reagents.
Practical/Tutorials. ABO and Rhesus grouping method
Antiserum Titration DCT and ICT antibody screening.
MLS 312: BASIC HAEMATOLOGY (3 Units)
Origin, development and function of blood cells. Synthesis and
breakdown of hemoglobin. Methods of Haemoglobin
estimation. Methods of cell counting. Absolute values.
Introduction to Homeostasis. Principles and mode of action of
common anticoagulants. Principle and components of
Hematological stains. Simple tests used in blood coagulation.
Blood films –normal and abnormal practical classes. Iron
metabolism, folate and Vit B 12 metabolism, Nomenclature,
classification and investigation of common
haemoglobinopathies, hemolytic anemia’s; myeloproferative
49
disorder; homeostasis and disorder of homeostasis;
investigation of bleeding disorders. Bone marrow. Practical
classes.
MLS 314: BASIC HISTOPATHOLOGY(3 Units)
Introduction to Histopathology, fixation autolysis bacterial
decomposition. Effects of fixation, common fixing agents and
their uses.Secondary fixation, post –fixation and post –
chroming and post mordating. Fixation, pigments
Decalcification. Dehydration, clearing and
infiltration/embedding. Frozen and celloidin sections.
Embedding media. Basic histology of organs. Principles and
application of exfoliativeCytology. Collection and fixation of
specimens for Cytological examination. Museum technique-
colour restoration. Mounting in museum jars. Tissues and
cellular injury inflammation. Healing and repairs. Gross post-
mortem slide examination to illustrate normal and abnormal
features appearance of diseased organs in routine and common
tumours.
MLS 327: LABORATORY MANAGEMENT
&ORGANIZATION (2 Units)
Laboratory Management, planning a medical laboratory
including the provision for the reception of patient’s selection
and storage of chemicals, materials and apparatus. Detailed
knowledge of the principles, use and maintenance of common
laboratory apparatus and equipment. Ventilation, air
conditioning and dust control in the laboratory. Equipment
used in special workbench e.g cutting—up benches media-
pouring, etc sterilization of air. Laboratory hazards and safety
measures to be taken in the use of radioactive and dangerous
materials. Emergency treatment for accidents. Laboratory
Records Maintenance of records: reception, recording storage,
filling and indexing of specimens and result. Organization and
operation of a system of quality control. Supply Chain
Management: Introduction to commodity supplies, SOP
50
Manual for facilities and staff in SCM, Cataloging and
Indexing of Laboratory supplies, Introduction to LMIS, Max-
Min, Inventory control system, Adjust Max-Min level.
Inventory, storage and distribution of health commodities,
Assessing health logistics, system selection, quantification of
Medical laboratory and Health commodities, Supply, Planning
and Shipment. Introduction to Medical Laboratory
commodities procurement, monitory and supervision. Methods
of recording experiments.
PCO 321: BASIC PHARMACOLOGY AND
TOXICOLOGY (2 Units)
Pharmacological terms. Drugs, sources and nature including
structure/activity relation. Bioassays. Routes of administration,
absorption, biotransformation and Elimination. Drug receptors
and receptor isolation. Fluorescent, radiosotopic and
chromatographic methods in drug studies. Methods of
evaluation of toxins, mutagens and carcinogens.
PCO 322: BASIC PHARMACOLOGY AND
TOXICOLOGY- PRACTICAL (1 unit)
Practical exercises on the topics thought in Basic
Pharmacology & Toxicology
400 LEVEL COURSES:
MLS 411: LABORATORY POSTING AND
PRACTICALS III (3 Units)
Posting of students to all sections of routine Medical
Laboratories for on-the-job training under the supervision of
qualified Medical Laboratory Scientists, for 2 days per week in
the entire semester. Scored logbook records per bench are kept
for each student per posting.
51
MLS 412: MEDICAL PARASITOLOGY AND
ENTOMOLOGY(3 Units)
Introduction to the parasites. Classification of protozoa, (the
amoebas, the ciliates, the flagellates, Nematodes. (Ascaris,
Strongyloidies, Trichuris, Guinea worms,
TrichinellaEnterobius, etc.).Life cycle and pathogenicity of
cestodes. (The tapeworms, larval forms of Cestodes). Life
cycle and pathogenicity of the Trematodes (The Schistosome,
Fasciola, paragonimusetc.). methods of demonstration of
parasite in blood, faces, vagina,urine urethra, pus from lung
and liver, skin snips, etc Mechanisms of their disease
production; Epidemiology and control of parasitic diseases.
Arthropods of medical importance—the crustaceans
ArachnidaHexapoda, Myiasis, etc.-their biology, life cycles
and control. Life history as disease vectors various disease of
medical importance transmissible by insects. Biology of
mosquito in relation to transmission of malarial, filariasis and
viral infections etc.
MLS 413: BASIC MEDICAL BACTERIOLOGY AND
MYCOLOGY (3 Units)
Methods for the demonstration of bacterial form and structure.
Design and preparation of culture media. Sterilization and other
methods of bacterial control. Aseptic procedures and methods
for pure culture isolation, procedures for receiving, handling
and processing of clinical specimens. Antibiotic assay,
sensitivity test and chemotherapy. Plate reading. Principle and
techniques of anaerobic bacteriology. Methods of total and
viable counts. Stock culture preservation, quality control of
culture and media. Record-keeping in Bacteriology laboratory.
Staining techniques for spores, capsules and negative staining
procedure, wet preparation, motility tests. Introductory
mycology.
52
MLS 414: INTRODUCTION TO BLOOD GROUP
SYSTEMS & COMPATIBILITY TESTS (3 Units)
Blood groups –other blood groups e.g MNS, Duffy, Kell, Kidd
etc. grouping techniques and antibody screening, clinical
significance, serostatus. Antenatal serology – screening and
Titration (quantitation) compatibility procedures-different
methods, advantage and disadvantages, Blood Transfusion
reactions. -causes and types; investigation. Risks attendant in
blood transfusion- Diseases, anaphylactic, hemolytic and
allergic reactions. Screening of Donor blood for diseases.
Compatibility procedures- advantages and disadvantages.
Practical based on the above topics.
MLS 415: ANALYTICALCLINICAL CHEMISTRY (3
Units)
Principles of analytical techniques in clinical chemistry-
devising new techniques, biological trials and tests for
acceptability. Solid/dry phase chemistry, dipstick technology,
thin film technology immobilized enzymes – analytical
techniques for qualitative and quantitative determination of
enzymes, hormones, proteins, lipid, trace elements, non-protein
nitrogen, volumetric analysis -partition, adsorption, gel
filtration, ion exchange and gas liquid chromatography.
Electrochemical analysis- principles of potentiometric analysis.
Fractionation of proteins-fractional precipitation (salting out),
chromatographic and electrophoretic procedures.Protein
precipitants-mode of action and choice in analytical procedures.
MLS 416: NUCLEIC ACID BIOCHEMISTRY AND
BASIC CONCEPTS OF MOLECULAR BIOLOGY (2
Units)
Nomenclature of bases, nucleosides and nucleotides. Nucleic
acids. Hydrolysis of nucleic acids. Analysis of nucleotide
sequence in nucleic acids and its application in diagnosis of
53
diseases. Nucleic acid protein complexes. Genetic role,
structure and replication of DNA. Introduction to polymerase
chain reaction and its application in laboratory diagnosis.
MLS 417: Cytological techniques (1 unit)
Collection, selection and preparation of cytology specimens
(cervical smear, vaginal smear, bronchial aspirates, ascitic
fluids and other fluids). Cytology staining techniques, normal,
atypical and malignant cells. Cornification index. Maturation
index, progesterone/androgen effects.
MLS 421: LABORATORY POSTING IV AND
PRACTICALS(3 Units)
2 days weekly for the entire semester. Scored log books are
kept by each student per posting.
MLS 422: VIROLOGY (3 Units)
Morphology and life cycle of viruses, nomenclature and
classification of viruses – various methods. Reproduction and
multiplication of viruses, resistance, pathology, collection of
clinical specimens for viral culture. Culture methods for
isolation of viruses, purification, immunity, laboratory
diagnosis of viral infection. Haemaglutination test, CFT,
Neutralization test, systematic study of viral diseases.
Interferon, immunotherapy and chemotherapy in viral infection,
inclusion bodies and cytopathic effects. Viral and host
interactions and identifications, Viral vaccines and immune-
prophylaxis.
MLS 423: HISTOPATHOLOGY AND
MUSEUMTECHNIQUES (3 Units)
Principle of photochemical methods.DNA - demonstration by
Feulgen techniques. Silver impregnation methods. Genes and
genetic code. Tissue culture techniques; chromosome analysis.
Autoradiography – Definition and principle. Organization of a
medical Museum. Method of colour maintenance. Fixation and
54
storage of museum specimens. Special museum techniques e.g
Dawson’s Method. Principle of photography- Macro and
Microphotography. Preparation of stained sections for
microphotography. Preparation of specimens for macro
photography. Cytological normal cells. Histology of tissues.
Atypical and malignant cells. Collection of cytological smears
and processing and screening. Principles of general pathology.
Systemic pathology. Gastrointestinal tract. Urogental,
coetaneous. Principle of electron microscopy. Practical based
on the topics.
MLS 424: BIOMEDICAL ENGINEERING (3 Units)
Workshop practice. Principles of use, maintenance and repair
of common apparatus and laboratory equipment. Principle of
applied and general electronics. Circuit diagrams computer
programming. Improvisation. Glass blowing and construction
of simple laboratory equipment. Design techniques,
improvement on existing equipment, review and modifications
of laboratory methods.
MLS 425: BIOTECHNOLOGY AND BIOINFORMATICS
(2 Units)
General preparation and storage of reagents for diagnostic use.
Preparation and purification of antibody and antigen for
diagnostic tools. Monoclonal and polyclonal antibodies.
Concepts of vaccination. Preparation, purification and storage
of vaccine. Introduction to Mathematical and Computation
Genomics. Its application to medicine in general and laboratory
diagnosis specifically.
MLS 426: COUNSELING SKILLS (3 Units)
Definition of counseling, care and support, types of counseling
pre-test, post-test prevention primary or secondary, crisis
management, problem-solving, decision-making couple
spiritual and pastoral; who needs counseling Prospect/benefits
of counseling constraints in counseling, rewarding listening
skills, prevention and managing conflicts. Genetic counseling
55
including sickle cell trait in marriage, Blood donation
campaign HIV infection etc. Case studies.
MLS 427: IMMUNOLOGY/IMMUNOCHEMISTRY
(3 Units)
Immunoglobulin-Structure and infection. Gene Organization
and assembly. Mediators of cellular Immunity. Phagocytic cell-
Chemotaxis and effectors function of Macrophage and
Granulocytes. The complement system. Laboratory methods of
detection of antigens and antibodies. Autoimmunity, Tissue
and Graft reactions Immunotolerance, self and nonself,
Histocompatibility, Transplantation, Tumor Immunology,
Hypersensitivity and allergy.
PHA 421 Chemotherapy of Microbial
Diseases,Vaccines and Sera (2 Units)
Antibacterials/Antibiotics The sulphonamides and
Trimethoprim. The penicillins and cephalosporins.
Tetracyclines and Chloramphenicol. The
Aminoglycosides. The Macrolides etc. Miscellaneous
Antimicrobials, Polypeptides. Antifungal and antiviral
agents. Drugs used in the treatment of Tuberculosis and
Leprosy Vaccines and Sera
MLS 428: FIRST PROFESSIONAL EXAMINATION
500 LEVEL COURSES:
GENERAL COURSES FOR ALL THE STUDENTS
56
MLS 511: LABORATORY POSTING V (3
credits)
Each student undergoes on the bench training in the different
analytical techniques used in the area of specialization. The
students are to participate in the routine operation of the
laboratory. Log books are kept by each student under the
supervision of a qualified medical laboratory scientists.
MLS 512: SEMINAR (2 credits)
Students are to carry out intensive literature research and
present seminar on selected approved topics to the
Departmental colloquium. Each presentation will be for about
15 to 20 minutes followed by general discussion. The
presentation will be scored by the group of internal assessors
appointed by the department.
MLS 513: RESEARCH METHODOLOGY (3 Credits)
Introduction to research methodology. Collection of literature
review articles. Problem definition. Sampling techniques.
Experimental designs of medical and data public health studies.
Questionnaire design and data collection analysis.
Interpretation and utilization of research findings. The role of
research in health and social welfare. The need for institutional
and governmental ethnical clearance for some research Aims,
characteristics and application of biostatistics. Measures of
central tendencies and variation. Collection and presentation of
data. Probability sampling. Test of statistical. Significance.
Experimental designs and clinical trials. Other applications of
biostatistics to clinical and preventive medicine projects.
Research proposals and sourcing of funding for research
projects. Arts of scholarly publications, and instructional
design.
MLS 519: CYTOGENETICS (2 Credits)
57
Theory and practice of clinical cytogenetics. Chromosome
analysis, structure, organization and staining techniques.
Chromosomes in man Normal karyotype and chromosome
abnormalities. Mosaicism, trisomy, monosomy, translocation
Klinefelters and Turner’s syndromes, sex chromatins.
Inactivation of X –chromosome and sex determination. Genetic
diseases. Clones, mapping of autosomes, DNA synthesis, gene
in kindred segregation. X-linked inheritance. Chimeras. Genes
in families and population. Selection, pedigree analysis,
mutation and mutagens, Hardy Weinberg equation, genetic
drift, inbreed. Slide reporting. Philadephiaand Christ church
chromosomes.
MLS 521: LABORATORY POSTING VI (3 Credits)
Each student undergoes on the bench training in the different
analytical techniques used in the area of specialization. The
students are to participate in the routine operation of the
laboratory. Log books are kept by each student under the
supervision of a qualified medical laboratory scientists.
MLS 522: GENETICS AND MOLECULAR BIOLOGY
(2 Credits)
Genomic Gene purification and amplification, polymerase
chain reaction technique. Construction of genetic maps.
Biotechnology – recombinant DNA, Hybriodoma.
MLS 524: PROJECT (6 Credits)
A supervised research project on an approved topic to be
undertaken by each student for the partial fulfillment of the
BMLS degree requirement. Assessment of the project will be
by both oral defense and grading of the project content.
CLINICAL CHEMISTRY SPECIALTY
MLS 514: CARBOHYDRATE, PROTEIN AND LIPID
METABOLISM (3 Credits)
58
Carbohydrate metabolism and disorder. Pathophysiology of
diabetes mellitus. Diabetic ketoacidosis, Hyperosmolar non
ketotic coma, lactic acidosis, Glycogen storage diseases.
Insulinoma. Diagnostic criteria and Laboratory investigation.
Fasting Plasma glucose, random plasma glucose, glucose
tolerant test, pancreatic hormones and glycosylated hemoglobin.
Lipid lipoproteins and apoproteins structure, composition and
function, intravascular metabolism and catabolism of
lipoproteins. Disorders of lipid and lipoproteins. Lipid storage
diseases. Cardiovascular function test. Recent advance in
diagnosis of lipids disorders. Plasma proteins in health and
diseases. Definition, cause and investigation of Para protein;
Bence Jones proteinuria) and significance. Fractionalization of
proteins. Protein electrophoresis in health and diseases. Protein
degradation. Metabolic disorder and regulation of amino acid
metabolism.
MLS 515: RENAL, LIVER & NEUROCHEMISTRY (3
Credits)
Physiology of kidney, renal clearance and glomerular filtration
rate. Renal plasma flow, maximal tubular excretory and
reabsorptive capacity. Urea, creatinine and insulin clearance.
Concentration and dilution tests. Renal failure, azotaemia,
anurea, sodium loss in renal diseases. Aminoaciduria. Kidney
diseases and kidney function test. Urinalysis in health and
diseases. Features of hypernatraemia and hyponatraemia.
Investigation of water and electrolyte imbalance. Homoeostasis
in clinical chemistry. Acid-base balance.
The liver anatomy and physiology-an overview. Biosynthesis
of bilirubin, excretion of bile pigments. Jaundice anatomical
and physiological classification. Pigment excretion in jaundice.
Liver diseases and liver functions test to include congo red test
for amyloisis and faecal fat estimation. Biochemistry of
Neoplastic disorders. Diseases of the nervous system. Basic
neurochemistry, CSF–normal composition and changes in
diseases. Diseases of muscles.
59
MLS 516: CLINICAL ENZYMOLOGY (3 Credits)
Mechanics of Enzyme action and kinetics, Activation
repression phenomenon. Enzyme induction, inhibition,
purification and specificity. Clinical Enzymology; Coenzymes
and Isoenzymes in medicine, diagnosis importance of
isoenzymes in biotechnology.
MLS 517: NUTRITION AND CLINICAL
VITAMINOLOGY (2 Credits)
Vitamins History and biochemical functions. Chemistry and
metabolism of water and fats soluble vitamins. Their deficiency
states and physiological significance. Relationship with
hormones. Vitamin in health and diseases. Methods of analysis.
Trace elements-Bioavailability, biochemical function,
metabolism, and interaction. Hormonal control and methods of
analysis. Specific elements in health and diseases. Bone
diseases and investigation of bone disorders. Types, causes etc.
Causes and Investigation of nutritional disorders.
MLS 526: DRUG MONITORING, TOXICOLOGY AND
INBORN ERROR OF METABOLISM (3 Credits)
Introduction to assimilation, distribution, elimination and
excretion of drugs. Practical and theoretical aspect of poisoning.
Investigation of suspected cases of poisoning. Estimation of
blood alcohol, Salicylate sulphonamide, cyanide, oxygen, CO2,
ammonia and Detection of barbiturate, cocaine heroin, opium,
phenothiazine, methaqualoneetc in blood, urine, sweat,
aspirates, etc. Porphyrin, causes, symptoms and laboratory
investigation of porphyrinaemia, porphyria and porphyrinuria.
Haemoglobin, synthesis, Chemistry of Haemoglobinopathies,
Sulp Hb, CoHb, Met Hb. Definition, causes, consequences and
investigation of some inborn errors of metabolism;
Phenylketonuria, galactosemia fructose intolerance, Albinism,
aminoaciduria.
60
MLS 527: CLINICAL AND REPRODUCTIVE
ENDOCRINOLOGY (3 Credits)
Endocrine glands-organization. Cellular communication by
endocrine glands. Endocrine receptor binding control of
endocrine action. Endocrine glands functions; the
hypothalamus, the pituitary, the parathyroid, adrenal cortex,
adrenal medulla. The gonads and reproductive endocrinology.
Foeto-placental function. Endocrine control of metabolism and
endocrine diseases/disorder; water balance, insulin action
thyroid hormone and reproduction. Investigation of male and
female infertility.
MLS 528: TECHNIQUES IN CLINICAL CHEMISTRY
(3 Credits)
Analytical techniques, standardization and quality control.
Validation of assay. Birth of a new method, devising new
techniques. Biological trial and tests for acceptability. Solid/dry
phase chemistry. Dipstick technology, thin film technology.
Immobilized enzymes. Functional test in clinical chemistry.
Liver function test. Renal function test. Gastro intestinal
function test etc. Analytical techniques employed in qualitative
and quantitative. Determination of (1) Enzymes-phosphates.
Transaminases, Dehydrogenases, kinases (2) Hormones (3)
protein-Total proteins, Albumin and globulin specific protein
(4) Lipids-cholesterol, triglycerides glycerol, fatty acids and
lipoproteins. (5) Trace elements-Fe, Cu, Zn, Mg, selenium (6)
Non-protein nitrogen-Urea, creatinine, uric acid, amino acids
and ammonia, urinalysis, determination of urine specific
gravity, osmolarity, qualitative tests for protein, glucose and
reducing substances. Ketone bodies, bilirubin, urobilinogen and
blood. Haemoglobin and haemoglobin derivative in urine.
Spectroscopy of Haemoglobin and its derivative in blood and
urine. Astrup techniques. Chromatography, spectroscopy,
61
spectrophotometry and photometry, AAS, Flame Photometer,
(AES), Radioimmunoassay, ELISA and EIA.
HAEMATOLOGY AND BLOOD TRANSFUSION
SCIENCE SPECIALITY
MLS 531: HAEMOPOIESIS, HAEMOGLOBIN,
HAEMOGLOBINOPATHIES
&MYELOPROLIFERATIONS (3 Credits)
Erythropoiesis and Blood cell counts in health and diseases.
Blood indices. Anaemia, disorders of Iron metabolism, vitamin
B12 and Folate deficiencies, Haemochromatosis and related
storage disorders. The spleen and splenomegaly syndromes.
Drugs, chemical and the blood Haemoglobinopathy,
Haemoglobin genotype and phenotype.
Blood in infancy, childhood and pregnancy, Hereditary and
blood disorders. Blood in microbial infections. Identification of
blood parasites Immuno-Haematological disorders,
autoimmune diseases, thrombocytopenia, leucopenia,
Leukemia systemic and disseminated lupus erythematosus,
rheumatoid arthritis myelomatosis and order paraproteinaemia.
Preparation and cytology of blood and bone marrow films in
health and disease.
MLS 532: BLOOD GROUP SYSTEMS AND
COMPATIBILITY TESTS (3 Credits)
ABO and other blood groups- MNS, KELL, Kidd, Duffy,
Lewis, p-1 etc. Antenatal Serology; Hemolytic diseases of the
newborn.Type, etiology, antenatal and post natal management.
Blood group serology in paternity dispute. Haemolysin titration.
Absorption and elution techniques. Indication and complication
of blood transfusion. Red cell survival tests— radioisotope and
differential agglutination methods. Screening of blood donor
for infective agents – HIV, HBV, malaria, filarial,
trypanosomes, syphilis, etc. anonymous result in blood
62
grouping. False positive and false negative result in
compatibility testing. Preparation and standardization of AHG.
MLS 533: SEROLOGY AND BLOOD TRANSFUSION
SCIENCE (3 Credits)
Leucocytes and platelet antigen and antibody. Auto-
mmunization IgM, IgG, IgA antibodies. National Blood
Transfusion Service. Preparation of commercial quantities of
polyclonal antisera. Principles, uses and techniques of
producing monoclonal and polyclonal antibodies. Types of
blood substitutes and preservations. Preparations of blood
products. WHO standards in BGS. Red cells membrane
structure in relation to blood antigen locations.
MLS 542: ADVANCED HAEMATOLOGICAL
TECHNIQUES (3 Credits)
Principles and techniques of isoelectric focusing. Protein
separation of column chromatography. Finger printing,
principles and techniques. Purification of proteins and enzymes.
Ultracentrifugation and molecular weight determination.
Culture of blood cells and parasite. Leukocyte typing platelet
aggregation- principles and techniques. Radioisotopes in
Heamatology; Isotope labeling techniques, measurement of
radioactivity Fluorescent antibody techniques.
Radioimmunoassay, ELIZA, western blotting immune-
electrophoresis, competitive protein binding. Automation in
Haematology, Electrophoresis- starch agar gel and
polyacrylamide gel. Principle of polymerase chain reaction.
Cytochemicalprocedures. Lymphocyte Transformation Tests
and Paul—Bunnel’s Test.
MLS 544: ADVANCED BLOOD GROUP SEROLOGY
TECHNIQUES (3 Credits)
Techniques for emergency compatibility testing – low ionic
sucrose solution, spin coomb’s albumin special compatibility
techniques Exchange and extracorporeal blood transfusion.
63
Preparation of enzymes used in BGS. Forensic application of
BGS, Two stage Coomb’s technique Automation in BGS-
Group and cross matchings Techniques, autoanalysers for
antibodies and antigen detection and identification, etc.
MLS 545: COAGULATION AND FIBRINOLYSIS
STUDIES (3 Credits)
Platelet functions, normal and abnormal haemostasis,
measurement of bleeding time. Vascular integrity.
Coagulation factors, Assessment of coagulation time. One
stage prothrombin time, “Thrombotest” Thromboplastin
generation. Haemophilia state, assay of anti-haemophilic factor
(VIII), recalcificationtime.Fibrinolytic activities, rapid
demonstration of fibrinogen deficiency. Simple assessment of
fibrinolysis. General Principles underlying clotting factor
assay and measurement of fibrinolytic activity. Platelet
substitute solutions. Fibrin plates.Control of anticoagulant
therapy.
HISTOPATHOLOGY SPECIALTY
MLS 534: FUNDAMENTAL HISTOPATHOLOGY (3
Credits)
Fixation: Purpose and effect of fixative composition and uses
of fixatives and their respective action on tissue components.
Microscopic appearance of tissues after various methods of
fixation. Function and scope of secondary fixation, post
fixation and post mordantings. Knowledge of fixation of
tissues for histochemical methods to include freeze drying and
freeze drying substitutes. Decalcification - processing
techniques – paraffin wax, embedding media for mechanical
and manual processing. Microtomy – Microtomes
(manipulation and uses of rocking, rotary, sledge, freezing,
cryostat and ultra-microtomes), knives – selection and
maintenance for various microtomes, manual and mechanical
sharpening. Section cutting (techniques used with different
64
embedding media, attachment of sections to slides-frozen
section techniques method for rapid diagnosis.
MLS 535: SYSTEMIC HISTOPATHOLOGY (3 Credits)
This course exposes the students more into general pathology,
control of results and management of Histopathology
laboratory. More facts of Electron microscopy and
Autoradiography are highlighted. Principles of general
pathology applied to individual organs. Systemic Pathology.
Hypertensive heart disease, heart failure and cardiomyopathies.
Respiratory – Tuberculosis, pneumonia, Nephropathy
associated with infestations and infections. CNS, special senses.
Malignant lymphomas (non-Hodgkins and Hodgkins
lymphoma, Burkitts). Idiopathic-tropical splenomegaly
syndrome. Liver –cirrhosis liver cells carcinoma. Hepatitis.
Female reproductive organs –pelvic inflammatory
diseases.Cancer-cervical, trophoblast, ovarian. Skin leprosy,
kaposis sarcoma. Electron microscopy- preparation of materials
for electron microscopy. Toxicity of some reagents used in
Electron microscopy. Techniques involved in autoradiography,
Laboratory Management. Quality control and automation in
histopathology laboratory. Slide Reporting.
MLS 536: HISTOCHEMISTRY AND HISIOLOGICAL
TECHNIQUES (3 Credits)
Enzyme histochemistry and its diagnostic application. The
theory of stains and application, metallic impregnation and
various histochemical methods. The dye theory. Properties of
natural and synthetic dyes. Composition, preparation and
storage of staining reagents. Testing of reagents. Common
nuclear stain and counter stain for general tissue structures.
Staining methods to demonstrate elastic, connective tissues and
fibers. Toxicity of some reagents used as it applies to auto-
radiography, electron microscopy and ultra microtomy.
65
Suitable fixatives for use, processing techniques,
impregnation/embedding and slide preparation /interpretation.
MLS 548: MEDICAL CYTOLOGY (2 Credits)
Study of epithelial cells. Introduction/definition of medical
exfoliative cytology. Definitions and principle of exfoliative
cytological methods. Gynaecological and non
gynaecologicalcytology.Cytology of normal and malignant
cells. Diagnostics criteria for all malignancy. Kinds of tumours.
Methods of collection of samples for gynaecological & non
gynaecological. Types of fixatives used. Staining techniques
applied. Hormonal evaluation/assessment. Identification of
virus, parasites.bacteria and fungi in smears. Slide
identification/interpretations. Principle of liquid basal cytology.
Usefulness and advantages, disadvantages and diagnostic
application.
MLS 561: EMBALMENT SCIENCE AND MUSEUM
TECHNIQUES (2 Credits)
History and science of embalmment. Formalin based
embalmment techniques. Other methods of preservation of
dead, cryopreservation (history, procedure and applications)
and mummification (history, procedure and applications).
Different embalmment techniques and problems. Museum
mounting of whole organs, techniques, importance and
application. Factors affecting embalming fluids. Setting up a
mortuary/medical museum. Forensic pathology as it applies to
post mortem examination, recording of pathological changes of
organs and collection of clinical data during autopsy especially
as it relates to drowning poisoning, strangulation e.t.c. Practical
based on the above topics are adviced. Dogs/goats can be used
for practical exercise.
MLS 562: IMMUNOHISTOCHEMISTRY (2 Credits)
Immunohistochemistry/immunocytochemistry, basic principles,
staining procedures and techniques. Peroxidase and anti-
66
peroxidase major histocompatibility. Immuotyping of tumors,
proteins and other diseases. Antibody and antigen preparation
from cells and tissues. Human leucocytes antigen. Reading and
interpretation of immunohistochemical/ immunocytochemical
stained slides. Preparation and production of
immunohistochemical/immunocytological stains.
MLS 563: STAINS AND STAINING TECHNIQUES (3
Credits)
Rapid H&E Frozen section, grams techniques. Maccivello
techniques, phloxinetetrazine, ziehl nelson, Perl’s Prussian blue,
schmorl’s reaction, Masson Fontana, Feulgen Reaction,
Giemsa, H&E, Gordon and Sweets, Haem Van Gieson, P.A.S.,
Jone’sMathenamine Sliver, Congo Red, Verhoeff’s
MSB,PAS/Orange G.,Aldehydefuchsin, Heidenhains iron haem,
P.T.A.H., Alcian blue/PAS, Best’s Kossa, Oil Red O., Nile
bule method. Bieschosky’s method, Marsland, Glee’s method,
papannicolaou, Barr body count, Hormonal Evaluation Gynae.
MEDICAL MICROBIOLOGY SPECIALITY
MLS 537: SYSTEMATIC BACTERIOLOGY (3 Credits)
History of pathogenic microbiology. Normal body flora,
pathogens sources of infection, laboratory diagnosis and
identification of bacteria. The pyogenic cocci, (Staphi, Strep,
Pneumococci and Neisseriae).The Enterobacteriaeceae,
coliforms, gastroenteritis, samonellosis, Shigallosis, choleras,
Vibrios, Pseudomonae, Bacteriodesetc.TheHaemophlic bacillus
(haemophilus, Brucellae, Yersinia, Bordetellaetc.Anaerobic
Spore formers, Aerobic spore formers, (Bacillus, Clostridia,
Spirochetes, Mycobacterium), Rickettsiae, Chalamydiae
Mycoplasma, L-forms, Listeria, Erysipelothrix, Bartonella etc.
General pathology, epidemiology, features, diagnosis, control
and therapy anaerobiosis
67
MLS 538: ADVANCED ENTOMOLOGY(3 Credits)
Structure and classification of Arthropods of medical
importance. Diptera:- Families – Culicidea, Psychodidae,
Sunuliidae, Ceratopogonidae, Tabanidae, Muscidae,
Calliophoridae, Oestridae, Hemiptera: Families – Cimicidae,
Reduviidae. Anoplura: Family – PediculidaeSiphonaptera:
Families – Pubicidae, Ceratophillidae, Leptosylliae, tungidae.
Acerina:- Families- Ixodidae, Argasudae, Trombiculidac,
Sarcoptidae, Demodicidae, Dermanyssidae, Poroceohalidae,
Liunguatulidae.
The epidemiology and geographical distribution of human
diseases. Larval migrants. Group Spirochaetaccea Immune
reactions (serology).
MLS 539: PUBLIC HEALTH MICROBIOLOGY (3
credits)
General principles of microbial disease transmission –
waterborne, airborne, food borne, arthropod-borne and
contagious disease. Principles and techniques for water
treatment, waste-water disposal. Preventive measures in the
control of bacterial, parasitic and viral infections. Vaccines and
immunisation. Immunisation programme and schedule (EPI).
MLS 550: MEDICAL MYCOLOGY(3 credits)
General characteristics of fungi’s diseases, types of mycoses
and properties; opportunistic fungi, Diagnosis and
chemotherapy, Systemic mycoses (Cryptococcosis-
Blastomycoses,
Histoplasmosis,Coccidiodomycoses).Opportunistic mycoses
(candidiasis, Phycomycoses; sporotrichoses,
Chromoblastomycosis, etc.)Cutaneous mycoses-
Dermatophytoses.Superficialmycoses.General properties,
pathogenesis, diagnosis, epidemiology, control and recognition
of fungi.
68
MLS 564: MEDICAL VIROLOGY(3 credits)
The dermatropic and viscerotropic viruses. Smallpox, cowpox,
and vaccination; measles, rubella, chickenpox and shingles,
Herpes Viruses,Yellow fever, lassa fever, Hep A, B and C,
influenza, arbo viruses. The neurotropic viruses (rabies,
poliomyelitis, encephalitis, Lymphocytic Choriomeningitis
viruses, mumps, viral transformation and types of tumors and
viruses.Oncogen theory etc. Viral gastroenteritis,
miscellaneous, viruses, vaccines, production and immunization.
MLS 565: PHARMACEUTICAL MICROBIOLOGY AND
MICROBIAL GENETICS
(3 Credits)
Principle of Antibiotics and chemotherapy. Mode of bacterial
resistance to antibiotics. Sensitivity testing. Preparation of
antibiogramdics. Minimum inhibitory concentration of
antibiotics.Historyof antibiotics, mode of action, classification,
antibiotics assay, use of animal model in the study of microbial
infections. Evolution and inheritance mutation.Bacterial DNA
in hereditary and mutation. Molecular basis of mutation,
isolation of mutants. Bacteriophages, plasmids, episomes,
transposons and bacterial DNA transfers. Recombinant DNA
technology and its applications.
MLS 566: LABORATORY TECHNIQUES IN
MICROBIOLOGY (3 Credits)
Culture media (Different types, compounding from basic
constituent and preparation of media). Examination, cultivation
and identification of bacteria from different samples, pleural,
CSF, urine, sputum, ascitic fluid. Blood culture, High vaginal
swab, wound swabs, ear, eye, nasal and other swabs. Stool
bacteriology. Sputum bacteriology. Urine bacteriology.
Systemic fungal culture and identification. Semen analysis.
Special -serological tests. ASO, Widal, VDRL, Rheumatoid
factor. Complement fixation, neutralization, hemagglutination
tests for identification of micro-organisms. General

69
identification of microorganisms by animal inoculation.
Biochemical test for the identification of bacteria and fungi.
WARDS AND ACHIEVEMENTS
1. Dr. T. Y. RAHEEM, was awarded a USAID K4Health
grant for e-Learning research and Capacity Building
(2012 -2016)
2. Dr. Mrs. R. M. KOLAWOLE received a Doctorate grant
from University of Lagos. Grant worth ₦500,000.
LINKAGES AND COLLABORATIONS
The University has an outstanding MoU with some
notable Research Institutes and Hospitals in Nigeria,
namely:
S/N
ORGANISATION
STATUS

70
1
Nigerian Institute of Medical
Research (NIMR)
MoU
2
Lagos State University Teaching
Hospital (LASUTH)
MoU
3
Lagos State Health Service
Commission
MoU
4
Educational Advancement Centre
MoU
5
Commit Technology and Consult
Ltd.
MoU
6
Edustart Global Foundation
MoU
7
New Horizon Systems Solution
Ltd.
MoU
8
Nigerian Employers Consultative
Association (NECA)
Registered
Member
9
Nigerian Association of Small and
Medium Enterprises (NASME)
Registered
Member

71
PENALTIES FOR EXAMINATION
MALPRACTICES
S/N
Misconducts
Penalties
1.
Possession/copying of any written materials
relevant to the examination, tests and
assignments.
Rustication for
two semesters.
2.
Impersonation
Expulsion
3.
Plagiarism
Rustication for
one semester.
4.
Unauthorized access to examination materials
Expulsion
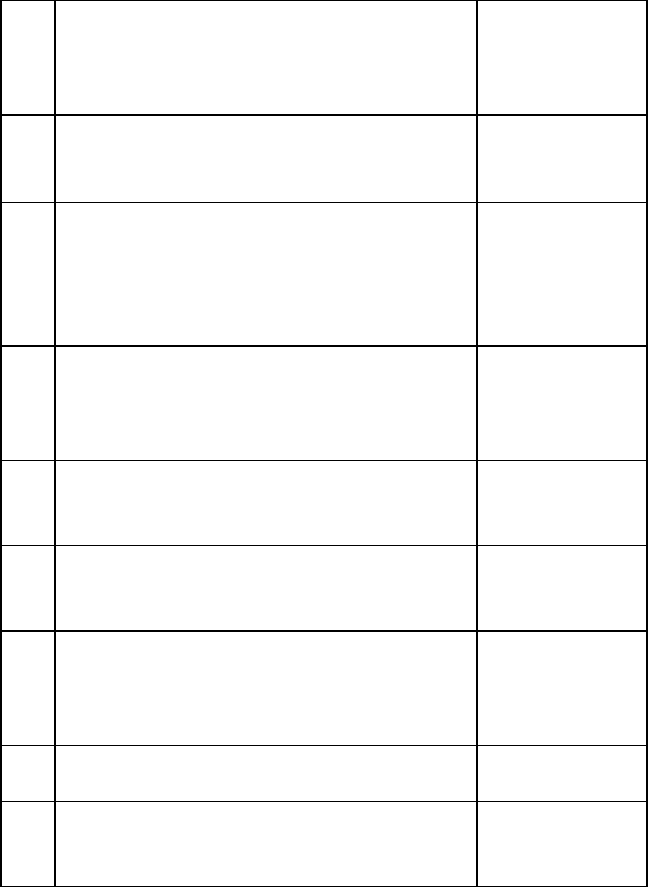
72
5.
Unauthorized collection of items from another
student during an examination without the
knowledge of the invigilator
Letter of caution
6.
Falsification of evaluation form
and other academic records or documents
Expulsion
7.
Appearing for examination, without meeting
attendance requirement
Letter of caution
and prevention
from writing the
examination.
8.
Disobedience to instructions/ disruption
during an examination/harassment of
invigilator
Disqualification
from the
examination.
9.
Harassment of Invigilators
Rustication for
one semester.
10.
Anti-safety behaviour during practical,
workshops, studio work, etc.
Letter of caution
11.
Attempted inducement of examiners and
invigilators
Disqualification
from the
examination
12.
Aiding and abetting examination misconduct
Expulsion.
13.
Destruction of evidence of examination
misconduct
Rustication for
one semester

73
14.
Refusal to complete examination misconduct
form
Rustication for
one semester.
15.
Any previous arrangement made for access to
examination materials whether it succeeds or
not
Rustication for
two semesters.
16.
Refusal to submit examination scripts
Failure in the
examined course.
17.
Any other misconduct recorded from time to
time
Penalty shall be
determined based
on the
recommendation
of the panel.
